Abstract
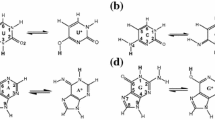
The interaction of diketo and keto-enol form of thymine and uracil tautomers with acridine (Acr), phenazine (Phen), benzo[c]cinnoline (Ben), 1,10-phenanthroline (1,10-Phe), and 4,7-phenenthroline (4,7-Phe) intercalating drug molecules was studied using density functional theory at B3LYP/6–311++G** and M05-2×/6–311++G** levels of theory. From the interaction energy, it is found that keto-enol form tautomers have stronger interaction with intercalators than diketone form tautomers. On complex formation of thymine and uracil tautomers with benzo[c]cinnoline the drug molecules have high interaction energy values of −20.14 (BenT3) and −20.55 (BenU3) kcal mol-1, while phenazine has the least interaction energy values of −6.52 (PhenT2) and −6.67 (PhenU2) kcal mol-1. The closed shell intermolecular type interaction between the molecules with minimum elliptical value of 0.018 and 0.019 a.u at both levels of theory has been found from topological analysis. The benzo[c]cinnoline drug molecule with thymine and uracil tautomers has short range intermolecular N-H…N, C-H…O, and O-H...N hydrogen bonds (H-bonds) resulting in higher stability than other drug molecules. The proper hydrogen bonds N-H..N and O-H..N have the frequency shifted toward the lower side (red shifted) with the elongation in their bond length while the improper hydrogen bond C-H...O has the frequency shifted toward the higher side (blue shifted) of the spectral region with the contraction in their bond length. Further, the charge transfer between proton acceptor and donor along with stability of the bond is studied using natural bond orbital (NBO) analysis.

Graphical abstract
Hydrogen bond interaction of diketo/keto-enol form uracil and thymine tautomers with intercalators





Similar content being viewed by others
References
Orozco M, López JM, Colomines C, Alhambra C, Busquets MA, Luque FJ (1995). J Am Chem Soc 117:1378–1386
Sayle RA (2010) So you think you understand tautomerism? J Comput Aided Mol Des 24:485–496
Fan JC, Shang ZC, Liang J, et al. (2010) Systematic theoretical investigations on the tautomers of thymine in gas phase and solution. J Mol Struct THEOCHEM 939:106–111
Rejnek J, Hanus M, Kabeláč M, Ryjáček F, Hobza P (2005) Correlated ab initio study of nucleic acid bases and their tautomers in the gas phase, in a microhydrated environment and in aqueous solution part 4 uracil and thymine. Phys Chem Chem Phys 7:2006–2017
Bodor N, Dewar MJS, Harget AJ (1970) Ground state of conjugated molecules XIX : tautomerism of heteroaromatic, hydroxy and amino derivatives and nucleotide bases. J Am Chem Soc 3217:2929–2936
Buda A, Sygula A (1983) MNDO study of the tautomers of nucleic bases part I. Uracil, thymine and cytosine. J Mol Struct THEOCHEM 92:255–265
Scanlan MJ, Hillier IH (1984) An ab Initio Study of the tautomerism of uracil thymine 5-fluorouracil and cytosine. J Am Chem Soc 106:3737–3745
Norinder U (1987) A theoretical reinvestigation of the nucleic bases adenine, guanine, cytosine, thymine and uracil using AM1. J Mol Struct THEOCHEM 151:259–269
Lest A, Adamowicz L (1989) Oxo-hydroxy tautomerism of uracil and 5-fluorouracll. J Phys Chem 85721:7078–7081
Orozco M, Hernandez B, Luque FJ (1998) Tautomerism of 1-methyl derivatives of uracil, thymine, and 5-bromouracil. Is tautomerism the basis for the mutagenicity of 5-bromouridine? J Phys Chem B 5647:5228–5233
Beak P (1977) Energies and alkylations of tautomeric heterocyclic compounds: old problems-new answers. Acc Chem Res 10:186–192
Rostkowska H, Szczepaniak K, Nowak MJ, Leszczynski J, Kubulat K, Person WB (1990) Tautomerism and infrared spectra of thiouracils. Matrix isolation and ab initio studies. J Am Chem Soc 112:2147–2160
Person WB, Szczepaniak K, Szczesniak M, Kwiatkowski JS, Hernandez L, Czerminski R (1989) Tautomerism of nucleic acid bases and the effect of molecular interactions on tautomeric equilibria. J Mol Struct 194:239–258
Zhang R, Ceulemans A, Nguyen MT (2005) A theoretical study of uracil and its tautomers in their lowest-lying triplet state. Mol Phys 103:983–994
Civcir PÜ (2000) A theoretical study of tautomerism of cytosine, thymine, uracil and their 1-methyl analogues in the gas and aqueous phases using AM1 and PM3. J Mol Struct THEOCHEM 532:157–169
Deepa P, Kolandaivel P (2012) Studies on Tautomeric forms of Guanine-Cytosine base pairs of nucleic acids and their interactions with water molecules. J Biomol Struct Dyn 25:733–746
Watson JD, Crick FHC (1969) Molecular structure of nucleic acids: a structure for deoxyribose nucleic acid (Reprinted from Nature, April 25, 1953). Nature 224:470–471
Ferguson LR, Denny WA (2007) Genotoxicity of non-covalent interactions: DNA intercalators. Mutat Res Fundam Mol Mech Mutagen 623:14–23
Hochstrasser RM (1962) Absorption spectrum of phenazine single crystals at 77° and 4.2°K in the Region of the n→π Transition. J Chem Phys doi:10.1063/1.1701271
Kallir AJ, Suter GW, Wild UP (1985) Multiple phosphorescence of phenazine in ethanol at 77 K. J Phys Chem 89:1996–1999
Aaron JJ, Maafi M, Párkányi C, Boniface C (1995) Quantitative treatment of the solvent effects on the electronic absorption and fluorescence spectra of acridines and phenazines. The ground and first excited singlet-state dipole moments. Spectrochim Acta A Mol Spectrosc 51:603–615
Hirata Y, Tanaka I (1976) Intersystem crossing to the lowest triplet state of phenazine following singlet excitation with a picosecond pulse. Chem Phys Lett 43:568–570
Grabowska A (1967) Triplet states of six-membered N-herterocycles spin-orbital coupling in diazines. Chem Phys Lett 1:113–116
Del Barrio JI, Rebato JR, G. -Tablas FM (1989) Basicity of phenazine in the first triplet state. J Phys Chem 93:6836–6837
Denny WA, Turner PM, Atwell GJ, et al. (1990) Structure-activity relationships for the mutagenic activity of tricyclic intercalating agents in Salmonella typhimurium. Mutat Res Fundam Mol Mech Mutagen 232:233–241
Vives M, Gargallo R, Tauler R (2000) Multivariate extension of the continuous variation and mole-ratio methods for the study of the interaction of intercalators with polynucleotides. Anal Chim Acta 424:105–114
Pilia L, Pizzotti M, Tessore F, Robertson N (2015) Tuning the LUMO energy of 1,10-phenanthroline in α-diimine–dithiolate Ni(II) complex and enhancement of nonlinear optical properties. Inorg Chim Acta 430:114–119
Choudhury SD, Basu S (2005) Interaction of phenazine with water and DNA bases. Spectrochim Acta A Mol Biomol Spectrosc 62:736–739
Sponer J, Jurecka P, Hobza P (2004) Accurate interaction energies of hydrogen-bonded nucleic acid base pairs. J Am Chem Soc 126:10142–10151
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Li YM, Zhou XM, Tang K, Qin M, Zhou ZY (2010) DFT studies on Hydrogen bonded complexes of thymine with formamide. Indian J Chem Sect A Inorg Phys Theor Anal Chem 49:145–150
Hall RJ, Hillier IH, Vincent MA (2000) Which density functional should be used to model hydration? Chem Phys Lett 320:139–143
Zhao Y, Schultz NE, Truhlar DG (2006) Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J Chem Theory Comput 2:364–382
Zhao Y, Truhlar DG (2007) Density functionals for noncovalent interaction energies of biological importance density functionals for noncovalent interaction energies of biological importance. J Chem Theory Comput 3:289–300
Boys S, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566
Popelier PLA, Bader W (1992) The existence of an intramolecular C-H-O hydroen bond in creatine and carbamoyl sarcosine. Chem Phys Lett 189:542–548
Cheeseman JR, Carroll MT, Bader RFW (1988) Themechanics of hydrogen bond formation in conjugated systems. Chem Phys Lett 143:450–458
MORPHY98 (1998) A program written by P.L.A. Popelier with a contribution from R.G.A Bone. UMIST, Manchester
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision A.1. Gaussian Inc, Wallingford
Rozas I (2007) On the nature of hydrogen bonds: an overview on computational studies and a word about patterns. Phys Chem Chem Phys 9:2782–2790
Dolgounitcheva O, Zakrzewski VG, Ortiz JV (1997) Ionization energies of acridine, phenazine, and diazaphenanthrenes. J Phys Chem A 101:8554–8564
Cubero E, Orozco M, Hobza P, Luque FJ (1999) Hydrogen bond versus anti-hydrogen bond: a comparative analysis based on the electron density topology. J Phys Chem A 103:6394–6401
Van der Veken BJ, Herrebout WA, Szostak R et al. (2001) The nature of improper, blue-shifting hydrogen bonding verified experimentally. J Am Chem Soc 123:12290–12293
Hobza P, Havlas Z (2000) Blue-shifting hydrogen bonds. Chem Rev 100:4253–4264
Bent HA (1960) distribution of molecules and atomic s character in its chemical implications. J Chem Educ 37:616–624
Shirley WA, Hoffmann R, Mastryukov VS (1995) Anapproach to understanding bond length/bond angle relationships. J Phys Chem 99:4025–4033
Burns LA, Vázquez-Mayagoitia Á, Sumpter BG, David Sherrill C (2011) Density-functional approaches to noncovalent interactions: a comparison of dispersion corrections (DFT-D), exchange-hole dipole moment (XDM) theory, and specialized functionals. J Chem Phys 134:084107
Singh SK, Kumar S, Das A (2014) Competition between n-πAr* and conventional hydrogen bonding (N–H…N) interactions: an ab initio study of the complexes of 7-azaindole and fluorosubstituted pyridines. Phys Chem Chem Phys 16:8819–8827
Shankar R, Radhika R, Thangamani D, Senthil Kumar L, Kolandaivel P (2014) Theoretical studies on interaction of anticancer drugs (dacarbazine, procarbazine and triethylenemelamine) with normal (AT and GC) and mismatch (GG, CC, AA and TT) base pairs. Mol Simul 7022:1–20
Popelier PLA (2000) Atoms in molecules: an introduction. Pearson, London
Bader RFW (1991) A quantum theory of molecular structure and its applications. Chem Rev 91:893–928
Paul BK, Mahanta S, Singh RB, Guchhait N (2010) A DFT-based theoretical study on the photophysics of 4-hydroxyacridine: single-water-mediated excited state proton transfer. J Phys Chem A 114:2618–2627
Popelier PLA (1998) Characterization of a dihydrogen bond on the basis of the electron density. J Phys Chem A 102:1873–1878
Yogeswari B, Kanakaraju R, Boopathi S, Kolandaivel P (2012) Microsolvation and hydrogen bond interactions in glycine dipeptide: molecular dynamics and density functional theory studies. J Mol Graph Model 35:11–20
Love I (2012) A QTAIM analysis of Cl, O bonds. Comput Theor Chem 985:23–29
Parthasarathi R, Subramanian V, Sathyamurthy N (2007) Hydrogen bonding in protonated water clusters: an atoms-in-molecules perspective. J Phys Chem A 111:13287–13290
Silva López C, Nieto Faza O, Cossió FP, et al. (2005) Ellipticity: a convenient tool to characterize electrocyclic reactions. Chem Eur J 11:1734–1738
Lu YX, Zou JW, Wang YH, et al. (2007) Ab initio investigation of the complexes between bromobenzene and several electron donors: Some insights into the magnitude and nature of halogen bonding interactions. J Phys Chem A 111:10781–10788
Arnold WD, Oldfield E (2000) The chemical nature of hydrogen bonding in proteins via NMR: J-couplings, chemical shifts, and AIM theory. J Am Chem Soc 122:12835–12841
Dunning Jr TH, Cartwright DC, Hunt WJ, et al. (1976) Generalized valence bond calculations on the ground state of nitrogen. J Chem Phys 64:4755
Niu X, Huang Z, Ma L, et al. (2013) Density functional theory, natural bond orbital and quantum theory of atoms in molecule analyses on the hydrogen bonding interactions in tryptophan-water complexes. J Chem Sci 125:949–958
Alabugin IV, Manoharan M, Peabody S, Weinhold F (2003) Electronic basis of improper hydrogen bonding: a subtle balance of hyperconjugation and rehybridization. J Am Chem Soc 125:5973–5987
Buemi G, Zuccarello F (2002) Is the intramolecular hydrogen bond energy valuable from internal rotation barriers? J Mol Struct 581:71–85
Ajayaghosh A (2003) Donor—acceptor type low band gap polymers: polysquaraines and related systems. Chem Soc Rev 32:181–191
Pearson RG (2005) Chemical hardness and density functional theory. J Chem Sci 117:369–377
Li AY, Wang SW (2007) Ab initio investigation of hydrogen bonds between pyridine and HCl, CHCl3. J Mol Struct THEOCHEM 807:191–199
Acknowledgments
SV expresses his sincere thanks to the Department of Science and Technology (DST-SERB), Government of India for the financial support in the form of a project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 744 kb)
Rights and permissions
About this article
Cite this article
Anithaa, V.S., Vijayakumar, S., Sudha, M. et al. Theoretical investigation on hydrogen bond interaction of diketo/keto-enol form uracil and thymine tautomers with intercalators. J Mol Model 23, 333 (2017). https://doi.org/10.1007/s00894-017-3476-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3476-8