Abstract
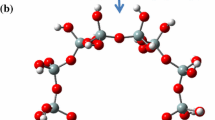
Hydrogen molecule adsorption on frameworks consisting of alkaline earth metal atoms (Be, Mg, or Ca) in LTL zeolite was investigated via density functional theory. A 24T zeolite cluster model was used in this study. HOMO and LUMO energy, chemical potential, chemical hardness, electronegativity, adsorption energy, and adsorption enthalpy values were calculated. The Mg-LTL and Ca-LTL clusters were found to have much lower chemical potentials and adsorption energies than those of the Be-LTL cluster. Additionally, the calculations indicated that the Mg-LTL and Ca-LTL clusters are softer (considering their lower chemical hardness values) and more chemically reactive than the Be-LTL cluster. The calculated hydrogen adsorption enthalpies were −14.7 and −9.4 kJ/mol for the Mg-LTL and Ca-LTL clusters, respectively, which are significantly larger than the enthalpy of liquefaction for the hydrogen molecule. These results imply that the Mg-LTL and Ca-LTL zeolite structures are promising cryoadsorbents for hydrogen storage.

Hydrogen adsorption was theoretically investigated on Be-, Ca- and Mg-LTL clusters. Ca- and Mg-LTL zeolites are potential cryoadsorbent materials for hydrogen storage.



Similar content being viewed by others
References
Strub AA, Imarisio G, Reidel D (1980) Hydrogen as an energy vector. Dordrecht
Du X, Huang Y, Zhang Q, Li J, Wu E (2012) Acta Pet Sin (Pet Process Sect) 28:137–140
Song MK, No KT (2007) Catal Today 120:374–382
Turnes Palomino G, Otero Areán C, Llop Carayol MR (2010) Appl Surf Sci 256:5281–5284
Langmi HW, Book D, Walton A, Johnson SR, Al-Mamouri MM, Speight JD, et al (2005) J Alloys Compd 404-406:637–642
Areán CO, Palomino GT, Carayol MRL, Pulido A, Rubeš M, Bludský O, et al (2009) Chem Phys Lett 477:139–143
Du XM, Huang Y, Wu ED (2011) Appl Mech Mater 55–57:1518–1522
Stephanie-Victoire F, Goulay AM, Cohen de Lara E (1998) Langmuir 14:7255–7259
Torres FJ, Civalleri B, Terentyev A, Ugliengo P, Pisani C (2007) J Phys Chem C 111:1871–1873
Turnes Palomino G, Llop Carayol MR, Otero Areán C (2008) Catal Today 138:249–252
Jhung SH, Sun Lee J, Woong Yoon J, Pyo Kim D, Chang JS (2007) Int J Hydrog Energy 32:4233–4237
Turnes Palomino G, Llop Carayol MR, Otero Areán C (2006) J Mater Chem 16:2884–2885
Otero Areán C, Turnes Palomino G, Llop Carayol MR (2007) Appl Surf Sci 253:5701–5704
Fellah MF (2017) Appl Surf Sci 394:9–15
Palomino GT, Bonelli B, Areán CO, Parra JB, Carayol MRL, Armandi M, et al (2009) Int J Hydrog Energy 34:4371–4378
Areán CO, Manoilova OV, Bonelli B, Rodríguez Delgado M, Turnes Palomino G, Garrone E (2003) Chem Phys Lett 370:631–635
Areán CO, Palomino GT, Garrone E, Nachtigallová D, Nachtigall P (2006) J Phys Chem B 110:395–402
Torres FJ, Vitillo JG, Civalleri B, Ricchiardi G, Zecchina A (2007) J Phys Chem C 111:2505–2513
Fellah MF (2014) J Porous Mater 21:883–888
Kohn W, Sham LJ (1965) Phys Rev 140:A1133–A1138
Frisch MF et al (2009) Gaussian 09. Gaussian Inc., Wallingford
Becke AD (1988) Phys Rev A 38:3098–3100
Lee C, Yang W, Parr R (1988) Phys Rev B 37:785–789
Kachurovskaya NA, Zhidomirov GM, Hensen EJM, Van Santen RA (2003) Catal Lett 86:25–31
Kachurovskaya NA, Zhidomirov GM, van Santen RA (2004) J Phys Chem B 108:5944–5950
Fellah MF (2011) J Phys Chem C 115:1940–1951
Rozanska X, Saintigny X, Van Santen RA, Hutschka F (2011) J Catal 202:141–155
Rozanska X, van Santen RA, Hutschka F, Hafner J (2011) J Am Chem Soc 123:7655–7667
Vos AM, Rozanska X, Schoonheydt RA, van Santen RA, Hutschka F, Hafner J (2001) J Am Chem Soc 123:2799–2809
Insuwan W, Rangsriwatananon K, Meeprasert J, Namuangruk S, Surakhot Y, Kungwan N, Jungsuttiwong S (2014) Micropor Mesopor Mat 197:348–357
Foresman JB, Frisch Æ (1996) Exploring chemistry with electronic structure methods, 2nd edn. Gaussian Inc., Pittsburgh, pp 68–69
Wong MW (1996) Chem Phys Lett 256:391–399
Parr RG, Yang W (1989) Density functional theory of atoms and molecules. Oxford University Press, New York
Kumar V, Shah EV, Roy DR (2015) Phys E 68:224–231
Pearson RG (2005) J Chem Sci 117:369–377
Pearson RG (1992) J Mol Struc (THEOCHEM) 255:261–270
Grimme S, Hujo W, Kirchner B (2010) Phys Chem Chem Phys 214:4875–4883
Andersson MP (2013) J Theor Chem 327839
Pearson RG (1998) Inorg Chem 27:734–740
Allred L (1961) J Inorg Nucl Chem 17:215–221
Kulkarni BS, Krishnamurty S, Pal S (2010) J Mol Catal A 329:36–43
Modi CK, Trivedi PM, Chudasama JA, Nakum HD, Parmar DK, Gupta SK, Jha PK (2014) Green Chem Lett Rev 7:278–287
Fellah MF (2016) Fuel Process Technol 144:191–196
Fallahpour F, Gorgani SS, Nouraliei M (2016) Indian J Phys 90(8):931–936
Samanta PN, Das KK (2016) Int J Quantum Chem 116:1467–1476
Ghamsari PA, Nouraliei M, Gorgani SS (2016) J Mol Graph Model 70:163–169
Perry RH, Green DW (1997) Section 2. In: Perry’s chemical engineers handbook, 7th edn. McGraw-Hill, Sydney
Acknowledgements
This work was supported by a research fund from Bursa Technical University (project number 2015-01-005). The numerical calculations reported in this paper were partially performed at TUBITAK-ULAKBIM, a high-performance and grid computing center (TRUBA resources).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 3.27 mb)
Rights and permissions
About this article
Cite this article
Fellah, M.F. A density functional theory study of hydrogen adsorption on Be-, Mg-, and Ca-exchanged LTL zeolite clusters. J Mol Model 23, 184 (2017). https://doi.org/10.1007/s00894-017-3349-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3349-1