Abstract
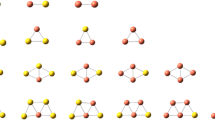
Density functional theory calculations were performed to investigate the adsorption behaviors of nitrogen molecule on small bimetallic AunCum and AunAgm clusters, with n + m ≤ 5. In all cases the N2 forms a linear or quasi-linear M-N-N structure (M = Au, Cu or Ag). The adsorption energies of N2 on pure metal clusters follow the order CunN2 > AunN2 > AgnN2, which is due to the weaker orbital interaction between silver and N2. N2 prefers to bind to a copper atom in AunCumN2 complexes and prefers to bind to a silver atom in AunAgmN2 complexes. The combination of Cu atoms into Aun clusters makes the cluster more reactive toward N2 while the combination of Ag atoms into Aun clusters makes the cluster less reactive toward N2. The electrostatic interaction is strengthened while the back-donation from metal to N2 is reduced in bimetallic cluster nitrides, as compared to the mono cluster nitrides. The N-N stretching frequencies are all red-shifted upon adsorption and the M-N stretching frequencies are highly correlated to the atoms to which the N is attached.










Similar content being viewed by others
References
Ervin KM (2001) Metal-ligand interactions: gas-phase transition metal cluster carbonyls. Int Rev Phys Chem 20:127–164
Zhang H, Zelmon DE, Deng L, Liu HK, Teo BK (2001) Optical limiting behavior of nanosized polyicosahedral gold-silver clusters based on third-order nonlinear optical effects. J Am Chem Soc 123:11300–11301
Lee HM, Ge M, Sahu BR, Tarakeshwar P, Kim KS (2003) Geometrical and electronic structures of gold, silver, and gold-silver binary clusters: origins of ductility of gold and gold-silver alloy formation. J Phys Chem B 107:9994–10005
Wang HQ, Kuang XY, Li HF (2010) Density functional study of structural and electronic properties of bimetallic copper-gold clusters: comparison with pure and doped gold clusters. Phys Chem Chem Phys 12:5156–5165
Zhao YR, Kuang XY, Zheng BB, Li YF, Wang SJ (2011) Equilibrium geometries, stabilities, and electronic properties of the bimetallic M2-doped Aun (M = Ag, Cu; n = 1–10) clusters: comparison with pure gold clusters. J Phys Chem A 115:569–576
Kuang X, Wang X, Liu G (2011) Structural, electronic and magnetic properties of small gold clusters with a copper impurity. Trans Met Chem 36:643–652
Jiang ZY, Lee KH, Li ST, Chu SY (2006) Structures and charge distributions of cationic and neutral Cun-1Ag clusters (n = 2-8). Phys Rev B 73:235423
Lou XH, Gao H, Wang WZ, Xu C, Zhang H, Zhang ZJ (2010) A theoretical study of the atomic hydrogen binding on small AgnCum (n + m ≤ 5) clusters. J Mol Struct (Theochem) 959:75–79
Zhao S, Lu WW, Ren YL, Wang JJ, Yin WP (2012) Density functional study of cationic and anionic AgmCun (m + n ≤ 5) clusters. Commun Theor Phys 57:452–458
Joshi AM, Delgass WN, Thomson KT (2006) Analysis of O2 adsorption on binary-alloy clusters of gold: energetics and correlations. J Phys Chem B 110:23373–23387
Liu X, Wang A, Wang X, Mou CY, Zhang T (2008) Au-Cu alloy nanoparticles confined in SBA-15 as a highly efficient catalyst for CO oxidation. Chem Commun 27:3187–3189
Wu DY, Ren B, Tian ZQ (2006) Binding interactions and Raman spectral properties of pyridine interacting with bimetallic silver-gold clusters. ChemPhysChem 7:619–628
Zhao Y, Li Z, Yang J (2009) A density functional study on cationic AunCum + clusters and their monocarbonyls. Phys Chem Chem Phys 11:2329–2334
Zhao S, Ren YL, Wang J, Yin WP (2010) Density functional study of hydrogen binding on gold and silver-gold clusters. J Phys Chem A 114:4917–4923
Arve K, Adam J, Simakova O, Capek L, Eranen K, Murzin DY (2009) Selective catalytic reduction of NOx over nano-sized gold catalysts supported on alumina and titania and over bimetallic gold-silver catalysts supported on alumina. Top Catal 52:1762–1765
Bin F, Song CL, Lv G, Song JO, Wu SH, Li XD (2014) Selective catalytic reduction of nitric oxide with ammonia over zirconium-doped copper/ZSM-5 catalysts. Appl Catal B 150:532–543
Kameoka S, Ukisu Y, Miyadera T (2000) Selective catalytic reduction of NOx with CH3OH, C2H5OH and C3H6 in the presence of O2 over Ag/Al2O3 catalyst: Role of surface nitrate species. Phys Chem Chem Phys 2:367–372
Ueda A, Haruta M (1999) Nitric oxide reduction with hydrogen, carbon monoxide, and hydrocarbons over gold catalysts. Gold Bull 32:3–11
Valden M, Lai X, Goodman DW (1998) Onset of catalytic activity of gold clusters on titania with the appearance of nonmetallic properties. Science 281:1647–1650
Özbek MO, Santen RAV (2013) The mechanism of ethylene epoxidation catalysis. Catal Lett 143:131–141
Yang LJ, Zhou S, Ding T, Meng M (2014) Superior catalytic performance of non-stoichiometric solid solution Ce1-xCuxO2-delta supported copper catalysts used for CO preferential oxidation. Fuel Process Technol 124:155–164
Maiti M, Sadhukhan D, Thakurta S, Zangrando E, Pilet G, Signorella S, Bellu S, Mitra S (2014) Catalytic efficacy of copper (II)- and cobalt (III)-Schiff base complexes in alkene epoxidation. Bull Chem Soc Jpn 87:724–732
Ding X, Yang J, Hou JG, Zhu Q (2005) Theoretical study of molecular nitrogen adsorption on Au clusters. J Mol Struct (Theochem) 755:9–17
Zhou J, Li ZH, Wang WN, Fan KN (2006) Density functional study of the interaction of carbon monoxide with small neutral and charged sliver clusters. J Phys Chem A 110:7167–7172
Cao Z, Wang J, Zhu J, Wu W, Zhang Q (2002) Static polarizabilities of copper clusters monocarbons CunCO (n = 2-13) and selectivity of CO adsorption on Copper Clusters. J Phys Chem B 106:9649–9654
Wu L, Senapati L, Nayak SK, Selloni A, Hajaligol M (2002) A density functional study of carbon monoxide adsorption on small cationic, neutral, and anionic gold clusters. J Chem Phys 117:4010–4015
Neumaier M, Weigend F, Hampe O, Kappes MM (2008) Binding energy and preferred adsorption sites of CO on gold and silver-gold cluster cations: adsorption kinetics and quantum chemical calculations. Faraday Discuss 138:393–406
Neumaier M, Weigend F, Hampe O, Kappes MM (2006) Reactions of mixed silver-gold cluster cations AgmAun + (m + n = 4,5,6) with CO: radiative association kinetics and density functional theory computations. J Chem Phys 125:104308
Joshi AM, Tucker MH, Delgass TKT (2006) CO adsorption on pure and binary-alloy gold clusters: a quantum chemical study. J Chem Phys 125:194707
Acioli PH, Burkland S, Srinivas S (2012) An exploration of the potential energy surface of the seven atom silver cluster and a carbon monoxide ligand. Eur Phys J D 66:215
31 Frisch MJ, Trucks GW, Schlegel HB et al. (2009) Gaussian 09, Gaussian Inc, Pittsburgh, PA
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1992) Atoms, molecules, Atoms, molecules, solids, and surfaces–Applications of the generalized gradient approximation for exchange and correlation. Phys Rev B 46:6671–6687
Andrae D, Haussermann U, Dolg M, Stoll H, Preuss H (1990) Energy–adjusted abinitio pseudopotentials for the 2nd and 3rd row transition–elements. Theor Chim Acta 77:123–141
Zhao S, Lu WW, Yun-Lai R, Yun-Li R, Wang J, Yin WP (2012) Density functional study of NOx binding on small AunCum (n + m ≤ 5) clusters. Comput Theor Chem 993:1039–1048
Foster JP, Weinhold F (1980) Natural hybrid orbitals. J Am Chem Soc 102:7211–7218
Dapprich S, Frenking G (1995) Investigation of donor-acceptor interactions: a charge decomposition analysis using fragment molecular orbitals. J Phys Chem 99:9352–9362
Frenking G, Fröhlich N (2000) The nature of bonding in transition-metal compounds. Chem Rev 100:717–774
Zhao S, Lu WW, Yun-Lai R, Yun-Li R, Wang J, Yin WP (2012) Density functional study of NOx binding on small AunCum (n + m ≤ 5) clusters. Comput Theor Chem 993:90–96
Sadek MM, Wang L (2006) Effect of adsorption site, size and composition of Pt/Au bimetallic clusters on the CO frequency: a density functional theory theory. J Phys Chem A 110:14036–14042
Fernandez EM, Soler JM, Garzon IL, Balbas LC (2004) Trends in the structure and bonding of noble metal clusters. Phys Rev B 70:165403
Wesendrup R, Hunt T, Schwerdtfeger P (2000) Relativistic coupled cluster calculations for neutral and singly charged Au3 clusters. J Chem Phys 112:9356–9362
Autschbach J, Siekierski S, Seth M, Schwerdtfeger P, Schwarz WHE (2002) Dependence of relativistic effects on electronic configuration in the neutral atoms of d- and f-block elements. J Comput Chem 23:804–813
Zhang X, Ding X, Fu Q, Yang J (2008) Theoretical study of molecular nitrogen adsorption on Wn clusters. J Mol Struct (Theochem) 867:17–21
Lei XL (2010) Theoretical study of small Mo clusters and molecular nitrogen adsorption on Mo clusters. Chin Phys B 19:107103
45 Huber KP, Herzberg G (1979) Molecular spectra and molecular structure. IV. Constants of diatomic molecules. Reinhold, New York
Davidson ER, Kunze KL, Machado FB, Chakravorty S (1993) The transition-metal carbonyl bond. Acc Chem Res 26:628–635
Bagus PS, Hermann K, Bauschlicher CW (1984) On the nature of the bonding of the lone pair ligands to small metal-clusters. Ber Bunsen-Ges Phys Chem 88:302–303
Koutecky J, Pacchioni G, Fantucci P (1985) Sigma-Pi-contributions in metal-CO bond: a theoretical-study of RhCO and PdCO. Chem Phys 99:87–101
Baerends EJ, Rozendaal A (1986) In: Veillard A (ed) Quantum chemistry: the challenge of transition metals and coordination chemistry. Reidel, Dordrecht
Muller W, Bagus PS (1985) Vibrations of CO chemisorbed on metal-surfaces: cluster model studies. J Vac Sci Technol 3:1623–1626
Boys SF, Bernadi F (1970) Calculation of small molecular interactions by differences of separate total energies-some procedures with reduced errors. Mol Phys 10:553–556
52 Mogi K, Sakai Y, Sonoda T, Xu Q, Souma Y (2003) Geometries and electronic structures of group 10 and 11 metal carbonys cations, [M(CO)n]x+ (Mx+ = Ni2+, Pd2+, Pt2+, Cu+, Ag+, Au+; n = 1-4). J Phys Chem A 107:3812–3821
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 21,133,009 and No. 21,002,023).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, S., Tian, X., Liu, J. et al. Density functional study of molecular nitrogen adsorption on gold-copper and gold-silver binary clusters. J Mol Model 20, 2467 (2014). https://doi.org/10.1007/s00894-014-2467-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2467-2