Abstract
Quantum chemical calculations at B3LYP/aug-cc-pVTZ level about singlet N-heterocyclic carbene (NHC) ligands, imidazol-2-ylidene, imidazol-4-ylidene, pyrazol-3-ylidene and pyrazol-4-ylidene, and their protonated analogues show that they are considerably aromatic except for pyrazol-3-ylidene. This result is experimentally verified by approximately five thousand NHC transition metal complexes retrieved from the Cambridge Structural Database (CSD). CSD search discloses that NHCs can participate in π-stacking interactions, albeit scarce. Geometry-based HOMA and electronic aromaticity index FLU rather than NICS provide a satisfactory description of the bonding situations in NHC ligands. Singlet state of the normal NHC has electron-deficient aromaticity as compared to those of the abnormal and remote NHCs. Depending on the transition from the singlet to triplet state, NHCs become electron-deficient ligands except for remote NHC. Computational studies regarding electronic nature of free NHC ligands show that the π-electronic population of the formally vacant pπ orbital on the carbene atoms in abnormal and remote NHC is occurred as a result of the aromaticity of NHCs, not as a result of the direct electron donation from LP-orbitals of N atoms to carbene atom according to putative push-pull effect used in understanding the electronic stabilization of normal NHC. Increase in the aromaticity raises σ-donating ability of both imidazol- and pyrazol-based NHC ligands. Free abnormal and remote NHC ligands have higher σ-donation ability than normal NHC ligands. The lack of σ-donating ability of normal NHC is compensated by its relatively high π-accepting ability, whereas π-back donation abilities of abnormal and remote NHCs are prohibited by their almost fully occupied π-orbitals. Aromaticities of the triplet NHC ligands are higher than that of the lowest-lying triplet state of benzene. Increase in the aromaticity of NHC ligands decreases van der Waals shortening in TM-NHC bonds mainly due to diminishing dative character of these bonds.

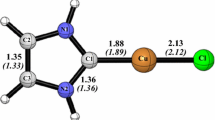
Singlet and triplet states of Arduengo type (normal) NHC showing their electron deficient aromatic characters






Similar content being viewed by others
References
Arduengo AJ III, Harlow RL, Kline MJ (1991) Am Chem Soc 113:361–363
Herrmann WA, Köcher C (1997) Angew Chem Int Ed Engl 36:2162–2187
Arduengo AJ III (1999) Acc Chem Res 32:913–921
Hahn FE, Jahnke MC (2008) Angew Chem Int Ed 47:3122–3172
Öfele K, Tosh E, Taubmann C, Herrmann WA (2009) Chem Rev 109:3408–3444
Wang Y, Robinson GH (2011) Inorg Chem 50:12326–12337
Al-Rafia SMI, Malcolm AC, Liew SK, Ferguson MJ, Rivard E (2011) J Am Chem Soc 133:777–779
Curran DP, Solovyev A, Makhlouf Brahmi M, Fensterbank L, Malacria M, Lacote E (2011) Angew Chem Int Ed 50:10294–10317
Wang Y, Robinson GH (2012) Dalton Trans 41:337–345
Kinjo R, Donnadieu B, Celik MA, Frenking G, Bertrand G (2011) Science 333:610–613
Martin D, Soleilhavoup M, Bertrand G (2011) Chem Sci 2:389–399
Schuster O, Yang L, Raubenheimer HG, Albrecht M (2009) Chem Rev 109:3445–3478
Nolan SP (2011) Acc Chem Res 44:91–100
Correa A, Nolan SP, Cavallo L (2011) Top Curr Chem 302:131–155
Valente C, Calimsiz S, Hoi KH, Mallik D, Sayah M, Organ MG (2012) Angew Chem Int Ed 51:3314–3332
Marion N, Diez-Gonzalez S, Nolan SP (2007) Angew Chem Int Ed 46:2988–3000
Biju AT, Kuhl N, Glorius F (2011) Acc Chem Res 44:1182–1195
Bugaut X, Glorius F (2012) Chem Soc Rev 41:3511–3522
Heinemann C, Müller T, Apeloig Y, Schwarz H (1996) J Am Chem Soc 118:2023–2038
Tomioka H (1997) Acc Chem Res 30:315–321
Hirai K, Itoh T, Tomioka H (2009) Chem Rev 109:3275–3332
Boehme C, Frenking G (1996) J Am Chem Soc 118:2039–2046
Lehmann JF, Urquhart SG, Ennis LE, Hitchcock AP, Hatano K, Gupta S, Denk MK (1999) Organometallics 18:1862–1872
Frison G, Sevin A (1999) J Phys Chem A 103:10998–11003
Diez-Gonzalez S, Nolan SP (2007) Coord Chem Rev 251:874–883
Muller P (1994) Pure Appl Chem 66:1077–1184
Leites LA, Magdanurov GI, Bukalov SS, Nolan SP, Scott NM, West R (2007) Mendeleev Commun 17:92–94
Arnold PL, Pearson S (2007) Coord Chem Rev 251:596–609
Huynh HV, Frison G (2013) J Org Chem 78:328–338
Aldeco-Perez E, Rosenthal AJ, Donnadieu B, Parameswaran P, Frenking G, Bertrand G (2009) Science 326:556–559
Lazzeretti P (2004) Phys Chem Chem Phys 6:217–223
Gomes JANF, Mallion RB (2001) Chem Rev 101:1349–1383
Bultinck P, Rafat M, Ponec R, van Gheluwe B, Carbo-Dorca R, Popelier P (2006) J Phys Chem A 110:7642–7648
Feixas F, Matito E, Poater J, Solà M (2008) J Comput Chem 29:1543–1554
Poater J, Fradera X, Duran M, Solà M (2003) Chem Eur J 9:400–406
Bultinck P, Ponec R, Van Damme SJ (2005) Phys Org Chem 18:706–718
Matito E, Duran M, Solà M (2005) J Chem Phys 122:014109–8
Becke AD (1993) J Chem Phys 98:5648–5652
Lee CT, Yang WT, Parr RG (1988) Phys Rev B 37:785–789
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision C01. Gaussian, Inc, Wallingford, CT
Dunning TH (1989) J Chem Phys 90:1007–1023
Woon DE, Dunning TH (1993) J Chem Phys 98:1358–1371
Glendening ED, Landis CR, Weinhold F (2012) WIREs Comput Mol Sci 2:1–42
Bader RFW (1994) Atoms in Molecules: A Quantum Theory. Oxford University Press, USA
Keith TA (2012) AIMAll (Version 12.09.23, Professional), TK Gristmill Software, Overland Park KS, USA, (aim.tkgristmill.com)
Mayer I (2007) J Comput Chem 28:204–221
Gorelsky SI, AOMix: Program for Molecular Orbital Analysis, http://www.sg-chem.net/, University of Ottawa, version 6.5, 2011
Krygowski TM, Cyrański MK (2001) Chem Rev 101:1385–1419
Chen ZF, King R (2005) Chem Rev 105:3613–3642
Cyrański MK (2005) Chem Rev 105:3773–3811
Ciesielski A, Krygowski TM, Cyrański MK, Dobrowolski MA, Balaban AT (2009) J Chem Inf Model 49:369–376
Krygowski TM (1993) J Chem Inf Comput Sci 33:70–78
Frizzo CP, Martins MAP (2012) Struct Chem 23:375–380
Matito E, Poater J, Duran M, Solà M (2005) J Mol Struc Theo chem 727:165–171
Bruno IJ, Cole JC, Edgington PR, Kessler M, Macrae CF, McCabe P, Pearson J, Taylor R (2002) Acta Crystallogr B58:389–397
Groom CR, Allen FH (2011) WIREs Comput Mol Sci 1:368–376
Holtzl T, Ngan VT, Nguyen MT, Veszprémi T (2009) Chem Phys Lett 481:54–57
Tonner R, Heydenrych G, Frenking G (2007) Chem Asian J 2:1555–1567
Parr RG, Von Szentpály L, Liu SB (1999) J Am Chem Soc 121:1922–1924
Minkin VI (1999) Pure Appl Chem 71:1919–1981
Feixas F, Vandenbussche J, Bultinck P, Matito E, Solà M (2011) Phys Chem Chem Phys 13:20690–20703
De Proft F, Geerlings P (2001) Chem Rev 101:1451–1464
Torrent-Sucarrat M, Luis JM, Duran M, Solà M (2002) J Chem Phys 117:10561–10570
Pearson RG (2005) J Chem Sci 117:369–377
Jacobsen H, Correa A, Poater A, Costabile C, Cavallo L (2009) Coord Chem Rev 253:687–703
Appelhans LN, Zuccaccia D, Kovacevic A, Chianese AR, Miecznikowski JR, Macchioni A, Clot E, Eisenstein O, Crabtree RH (2005) J Am Chem Soc 127:16299–16311
Crabtree RH (2013) Coord Chem Rev 257:755–766
Iglesias M, Albrecht M (2010) Dalton Trans 39:5213–5215
Albrecht M (2009) CHIMIA 63:105–110
Arduengo AJ, Dias HVR, Dixon DA, Harlow RL, Klooster WT, Koetzle TF (1994) J Am Chem Soc 116:6812–6822
Krygowski TM, Palusiak M, Plonka A, Zachara-Horeglad JE (2007) J Phys Org Chem 20:297–306
Frenking G, Solà M, Vyboishchikov SF (2005) J Organomet Chem 690:6178–6204
Heydenrych G, von Hopffgarten M, Stander E, Schuster O, Raubenheimer HG, Frenking G (2009) Eur J Inorg Chem 1892–1904
Parr RG, Chattaraj PK (1991) J Am Chem Soc 113:1854–1855
Heckenroth M, Neels A, Garnier MG, Aebi P, Ehlers AW, Albrecht M (2009) Chem Eur J 15:9375–9386
Cordero B, Gómez V, Platero-Prats AE, Revés M, Echeverría J, Cremades E, Barragán F, Alvarez S (2008) Dalton Trans 21:2832–2838
Baba E, Cundari TR, Firkin I (2005) Inorg Chim Acta 358:2867–2875
Fernandez I, Dyker CA, DeHope A, Donnadieu B, Frenking G, Bertrand G (2009) J Am Chem Soc 131:11875–11881
Bourissou D, Guerret O, Gabbai FP, Bertrand G (2000) Chem Rev 100:39–91
Martinez CR, Iverson BL (2012) Chem Sci 3:2191–2201
Janiak C (2000) J Chem Soc Dalton Trans 21:3885–3896
Acknowledgments
This work was supported by TÜBİTAK (The Scientific and Technological Research Council of Turkey) under the allocation 112T636.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1160 kb)
Rights and permissions
About this article
Cite this article
Sevinçek, R., Karabıyık, H. & Karabıyık, H. Changes in ligating abilities of the singlet and triplet states of normal, abnormal and remote N-heterocyclic carbenes depending on their aromaticities. J Mol Model 19, 5327–5341 (2013). https://doi.org/10.1007/s00894-013-2027-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-013-2027-1