Abstract
We report a density functional theory study on the electronic structure properties of pristine and phosphorous-doped (6,0) and (4,4) single-walled BC3 nanotubes (BC3NTs). We examine the usefulness of local reactivity descriptors to predict the reactivities of different carbon/boron atomic sites on the external surface of the tubes. Electrostatic potentials VS(r) and average local ionization energies ĪS(r) are computed on the surface of the investigated BC3NTs. A general feature of the systems considered here is that the magnitudes of negative VS(r) associated with carbon atoms tend to be stronger when the boron atom is substituted with a phosphorous atom. In order to verify the surface reactivity pattern based on the chosen reactivity descriptors, we calculated the reaction energies for the interaction of an H+ ion or H radical with external surface of the (6,0) and (4,4) BC3NTs. It is clear that, for each nanotube studied, the reaction energies correlate well with the values of VS(r) and ĪS(r).

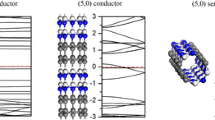
Structure and atomic numbering scheme for a pristine (6,0) BC3NT and b pristine (4,4) BC3NT





Similar content being viewed by others
References
Dinadayalane TC, Leszczynski J (2010) Remarkable diversity of carbon–carbon bonds: structures and properties of fullerenes, carbon nanotubes, and graphene. Struct Chem 21:1155–1169
Saha S, Dinadayalane TC, Leszczynska D, Leszczynski J (2012) Open and capped (5,5) armchair SWCNTs: a comparative study of DFT-based reactivity descriptors. Chem Phys Lett 541:85–91
Nojeh A, Lakatos GW, Peng S, Cho K, Pease RFW (2003) A carbon nanotube cross structure as a nanoscale quantum device. Nano Lett 3:1187–1190
Wang Y, Shao Y, Matson DW, Li J, Lin Y (2010) Nitrogen-doped graphene and its application in electrochemical biosensing. ACS Nano 4:1790–1798
Cruz-Silva E, López-Urías F, Muñoz-Sandoval E, Sumpter BG, Terrones H, Charlier JC, Meunier V, Terrones M (2010) Electronic transport and mechanical properties of phosphorus- and phosphorus nitrogen-doped carbon nanotubes. ACS Nano 3:1913–1921
Mykhailenko O, Matsui D, Prylutskyy Y, Le Normand F, Eklund P, Scharff P (2007) Monte Carlo simulation of intercalated carbon nanotubes. J Mol Model 13:283–287
Nikitin A, Li X, Zhang Z, Ogasawara H, Dai H, Nilsson A (2008) Hydrogen storage in carbon nanotubes through the formation of stable CH bonds. Nano Lett 8:162–167
Chen P, Wu X, Lin J, Tan K (1999) High H2 uptake by alkali-doped carbon nanotubes under ambient pressure and moderate temperatures. Science 285:91–93
Pan X, Fan Z, Chen W, Ding Y, Luo H, Bao X (2007) Enhanced ethanol production inside carbon-nanotube reactors containing catalytic particles. Nat Mater 6:507–511
Wang Z, Jia R, Zheng J, Zhao J, Li L, Song J, Zhu Z (2011) Nitrogen-promoted self-assembly of N-doped carbon nanotubes and their intrinsic catalysis for oxygen reduction in fuel cells. ACS Nano 5:1677–1684
Gong K, Du F, Xia Z, Durstock M, Dai L (2009) Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 323:760–764
Yang S, Zhao G, Khosravi E (2010) First principles studies of nitrogen doped carbon nanotubes for dioxygen reduction. J Phys Chem C 114:3371–3375
Xu X, Jiang S, Hu Z, Liu S (2010) Nitrogen-doped carbon nanotubes: high electrocatalytic activity toward the oxidation of hydrogen peroxide and its application for biosensing. ACS Nano 4:4292–4298
Cruz-Silva E, López-Urías F, Munõz-Sandoval E, Sumpter BG, Terrones H, Charlier JC, Meunier V, Terrones M (2009) Electronic transport and mechanical properties of phosphorus- and phosphorus nitrogen-doped carbon nanotubes. ACS Nano 3:1913–1921
Guo H, Kumar S, Hauge RH, Smalley RE (2004) Polyacrylonitrile single-walled carbon nanotube composite fibers. Adv Mater 16:58–61
Panchakarla LS, Govindaraj A, Rao CNR (2007) Nitrogen- and boron-doped double-walled carbon nanotubes. ACS Nano 1:494–500
Miyamoto Y, Rubio A, Louie SG, Cohen ML (1994) Electronic properties of tubule forms of hexagonal BC3. Phys Rev B 50:18360–18366
Su WS, Chang CP, Li MF, Li TL (2011) Electronic structures and work functions of BC3 nanotubes: a first-principle study. J Appl Phys 110:014312
Tománek D, Wentzcovitch RM, Louie SG, Cohen ML (1988) Calculation of electronic and structural properties of BC3. Phys Rev B 37:3134–3136
Wang Q, Chen LQ, Annett JF (1996) Stability and charge transfer of C3B ordered structures. Phys Rev B 54:R2271–R2275
Fuentes GG, Borowiak-Palen E, Knupfer M, Pichler T, Fink J, Wirtz L, Rubio A (2004) Formation and electronic properties of BC3 single-wall nanotubes upon boron substitution of carbon nanotubes. Phys Rev B 69:245403
Borowiak-Palen E, Rümmeli M, Gemming T, Knupfer M, Kalenczuk RJ, Pichler T (2005) Formation of novel nanostructures using carbon nanotubes as a frame. Synth Metal 153:345–348
Tanaka H, Kawamata Y, Simizu H, Fujita T, Yanagisawa H, Otani S, Oshima C (2005) Novel macroscopic BC3 honeycomb sheet. Solid State Commun 136:22–25
Herńandez E, Goze C, Bernier P, Rubio A (1998) Elastic properties of C and BxCyNz composite nanotubes. Phys Rev Lett 80:4502–4505
Ren X, Chen C, Nagatsu M, Wang X (2011) Carbon nanotubes as adsorbents in environmental pollution management: a review. Chem Eng J 170:395–410
Zhao J, Buldum A, Han J, Lu JP (2002) Gas molecule adsorption in carbon nanotubes and nanotube bundles. Nanotechnology 13(2):195–200
Shirvani BB, Beheshtian J, Esrafili MD, Hadipour NL (2010) DFT study of NH3 adsorption on the (5,0), (8,0), (5,5) and (6,6) single-walled carbon nanotubes calculated binding energies, NMR and NQR parameters. Phys B 405:1455–1460
Frank B, Zhang J, Blume R, Schlüogl R, Su D (2009) Heteroatoms increase the selectivity in oxidative dehydrogenation reactions on nanocarbons. Angew Chem Int Ed 48:6913–6917
Ayala P, Arenal R, Rüummeli M, Rubio A, Pichler T (2010) The doping of carbon nanotubes with nitrogen and their potential applications. Carbon 48:575–586
Lin YH, Lu F, Tu Y, Ren ZF (2004) Glucose biosensors based on carbon nanotube nanoelectrode ensembles. Nano Lett 4:191–195
Naray-Szabo G, Ferenczy GG (1995) Molecular electrostatics. Chem Rev 95:829–847
Sauer J, Ugliengo P, Garrone E, Saunders VR (1994) Theoretical study of van der Waals complexes at surface sites in comparison with the experiment. Chem Rev 94:2095–2160
Bulat FA, Toro-Labbé A, Brinck T, Murray JS, Politzer P (2010) Quantitative analysis of molecular surfaces: areas, volumes, electrostatic potentials and average local ionization energies. J Mol Model 16:1679–1691
Murray JS, Peralta-Inga Z, Politzer P (2000) Computed molecular surface electrostatic potentials of the nonionic and zwitterionic forms of glycine, histidine, and tetracycline. Int J Quantum Chem 80:1216–1223
Politzer P, Murray JS, Concha MC (2007) Halogen bonding and the design of new materials: organic bromides, chlorides and perhaps even fluorides as donors. J Mol Model 13:643–650
Riley KE, Murray JS, Politzer P, Concha MC, Hobza P (2009) Br · · · O complexes as probes of factors affecting halogen bonding: interactions of bromobenzenes and bromopyrimidines with acetone. J Chem Theory Comput 5:155–163
Esrafili MD (2012) Investigation of H-bonding and halogen-bonding effects in dichloroacetic acid: DFT calculations of NQR parameters and QTAIM analysis. J Mol Model 18:5005–5016
Esrafili MD (2013) A theoretical investigation of the characteristics of hydrogen/ halogen bonding interactions in dibromo-nitroaniline. J Mol Model 19:1417–1427
Peralta-Inga Z, Lane P, Murray JS, Boyd S, Grice ME, O’Connor CJ, Politzer P (2003) Characterization of surface electrostatic potentials of some (5,5) and (n,1) carbon and boron/nitrogen model nanotubes. Nano Lett 3:21–28
Politzer P, Lane P, Murray JS, Concha MC (2005) Comparative analysis of surface electrostatic potentials of carbon, boron/nitrogen and carbon/boron/nitrogen model nanotubes. J Mol Model 11:1–7
Politzer P, Murray JS, Lane P, Concha MC, Jin P, Peralta-Inga Z (2005) An unusual feature of end-substituted model carbon (6,0) nanotubes. J Mol Model 11:258–264
Esrafili MD, Behzadi H (2013) A comparative study on carbon, boron-nitride, boron-phosphide and silicon-carbide nanotubes based on surface electrostatic potentials and average local ionization energies. J Mol Model 19:2375–2382
Esrafili MD (2013) Nitrogen-doped (6, 0) carbon nanotubes: a comparative DFT study based on surface reactivity descriptors. Comput Theor Chem 1015:1–7
Dinadayalane TC, Murray JS, Concha MC, Politzer P, Leszczynski J (2010) Reactivities of sites on (5,5) single-walled carbon nanotubes with and without a Stone-Wales defect. J Chem Theory Comput 6:1351–1357
Saha S, Dinadayalane TC, Murray JS, Leszczynska D, Leszczynski J (2012) Surface reactivity for chlorination on chlorinated (5,5) armchair SWCNT: a computational approach. J Phys Chem C 116:22399–22410
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) General atomic and molecular electronic structure system. J Comput Chem 14:1347–1363
Politzer P, Murray JS, Sen KD (1996) Molecular electrostatic potentials: concepts and applications. Elsevier, Amsterdam
Sjoberg P, Murray JS, Brinck T, Politzer P (1990) Average local ionization energies on the molecular surfaces of aromatic systems as guides to chemical reactivity. Can J Chem 68:1440–1443
Politzer P, Murray JS, Bulat FA (2010) Average local ionization energy: a review. J Mol Model 16:1731–1742
Politzer P, Murray JS, Peralta-Inga Z (2010) Molecular surface electrostatic potentials in relation to noncovalent interactions in biological systems. Int J Quantum Chem 85:676–684
Brinck T, Murray JS, Politzer P (1992) Quantitative determination of the total local polarity (charge separation) in molecules. Mol Phys 76:609–617
Esrafili MD (2013) Electronic structure and surface reactivity of BC3 nanotubes from first-principle calculations. Struct Chem. doi:10.1007/s11224-013-0269-2
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alizadeh, M., Esrafili, M.D. & Vessally, E. Exploring surface reactivity of phosphorous-doped (6,0) and (4,4) BC3 nanotubes: a DFT study. J Mol Model 19, 4877–4886 (2013). https://doi.org/10.1007/s00894-013-1978-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-013-1978-6