Abstract
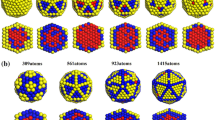
The properties of noble gas systems can be greatly extended by heterogeneous mixtures of elements. The geometrical structures and energies of mixed Ar–Kr–Xe clusters were investigated using ternary Lennard-Jones (TLJ) potential. For the Ar19Kr n Xe19, Ar19Kr19Xe n , and Ar n Kr19Xe19 (n = 0–17) clusters investigated, the results show that only two minimum energy configurations exist, i.e., polytetrahedron and six-fold pancake. The inner core of all these clusters is composed mainly of Ar atoms, and Kr and Xe atoms are distributed on the surface with well mixed pattern for polytetrahedral and segregate pattern for six-fold pancake configurations. The relative stability property of Ar–Kr–Xe clusters with a certain composition is discussed. Moreover, the role of heterogeneity on the strain was investigated, and reduced strain energies in Ar–Kr–Xe clusters were studied to find possible ways of reducing strain. The results showed that the strain energies were affected mainly by Ar–Ar, Ar–Kr, and Xe–Xe bonds.

Investigation of the structures of Ar19Kr n Xe19, Ar19Kr19Xe n , and Ar n Kr19Xe19 (n = 0–17) clusters reveal the existence of only exist two minimum energy configurations, i.e., polytetrahedron and six-fold pancake. Furthermore, reduced strain energies in Ar–Kr–Xe clusters were studied for the possible ways of reducing strain.





Similar content being viewed by others
References
Castleman AW Jr, Keesee RG (1988) Gas-phase clusters: spanning the states of matter. Science 241:36–42
Bordner AJ (2012) Assessing the accuracy of SAPT(DFT) interaction energies by comparison with experimentally derived noble gas potentials and molecular crystal lattice energies. ChemPhysChem 13:3981–3988
Lee JW, Stein GD (1987) Structure change with size of argon clusters formed in laval nozzle beams. J Phys Chem 91:2450–2457
Zweiback J, Ditmire T, Perry MD (1999) Femtosecond time-resolved studies of the dynamics of noble-gas cluster explosions. Phys Rev A 59:3166–3169
Miehle W, Kandler O, Leisner T, Echt O (1989) Mass spectrometric evidence for icosahedral structure in large rare gas clusters: Ar, Kr, Xe. J Chem Phys 91:5940–5952
Rolles D, Zhanga H, Pešić ZD, Bozek JD, Berrah N (2009) Emergence of valence band structure in rare-gas clusters. Chem Phys Lett 468:148–152
Cybulski SM, Toczyłowski RR (1999) Ground state potential energy curves for He2, Ne2, Ar2, He–Ne, He–Ar, and Ne–Ar: a coupled-cluster study. J Chem Phys 111:10520–10528
Jeziorska M, Cencek W, Patkowski K, Jeziorski B, Szalewicz K (2007) Pair potential for helium from symmetry-adapted perturbation theory calculations and from supermolecular data. J Chem Phys 127:124303
Williams HL, Korona T, Bukowski R, Jeziorski B, Szalewicz K (1996) Helium dimer potential from symmetry-adapted perturbation theory. Chem Phys Lett 262:431–436
Patkowski K, Szalewicz K (2010) Argon pair potential at basis set and excitation limits. J Chem Phys 133:094304
Pillardy J, Olszewski KA, Piela L (1992) Performance of the shift method of global minimization in searches for optimum structures of clusters of Lennard-Jones atoms. J Phys Chem 96:4337–4341
Hermann A, Schwerdtfeger P (2009) Complete basis set limit second-order Møller-Plesset calculations for the fcc lattices of neon, argon, krypton, and xenon. J Chem Phys 131:244508
Doye JPK, Meyer L (2005) Mapping the magic numbers in binary Lennard-Jones clusters. Phys Rev Lett 95:063401
de Souza VK, Wales DJ (2009) Connectivity in the potential energy landscape for binary Lennard-Jones systems. J Chem Phys 130:194508
Wu X, Sun Y, Li CS, Yang W (2012) Parametric effects of the potential energy function on the geometrical features of ternary Lennard-Jones clusters. J Phys Chem A 116:8218–8225
Dieterich JM, Hartke B (2011) Composition-induced structural transitions in mixed Lennard-Jones clusters: global reparametrization and optimization. J Comput Chem 32:1377–1385
Marques JMC, Pereira FB (2013) A detailed investigation on the global minimum structures of mixed rare-gas clusters: geometry, energetics, and site occupancy. J Comput Chem 34:505–517. doi:10.1002/jcc.23161
Northby JA (1987) Structure and binding of Lennard-Jones clusters: 13 ≤ N ≤ 147. J Chem Phys 87:6166–6177
Romero D, Barron C, Gomez S (1999) The optimal geometry of Lennard-Jones clusters: 148–309. Comput Phys Commun 123:87–96
Xiang YH, Jiang HY, Cai WS, Shao XG (2004) An efficient method based on lattice construction and the genetic algorithm for optimization of large Lennard-Jones clusters. J Phys Chem A 108:3586–3592
Xiang YH, Cheng LJ, Cai WS, Shao XG (2004) Structural distribution of Lennard-Jones clusters containing 562 to 1000 atoms. J Phys Chem A 108:9516–9520
Hu M, Shenogin S, Keblinski P (2007) Thermal energy exchange between carbon nanotube and air. Appl Phys Lett 90:231905
Johnston RL (2003) Evolving better nanoparticles: genetic algorithms for optimising cluster geometries. J Chem Soc Dalton Trans 22:4193–4207
Hsu PJ, Lai SK (2006) Structures of bimetallic clusters. J Chem Phys 124:044711
Kim HG, Choi SK, Lee HM (2008) New algorithm in the basin hopping Monte Carlo to find the global minimum structure of unary and binary metallic nanoclusters. J Chem Phys 128:144702
Wu X, Cai WS, Shao XG (2009) Optimization of bimetallic Cu–Au and Ag–Au clusters by using a modified adaptive immune optimization algorithm. J Comput Chem 30:1992–2000
Wu X, Wu GH, Chen YC, Qiao YY (2011) Structural optimization of Cu–Ag–Au trimetallic clusters by adaptive immune optimization algorithm. J Phys Chem A 115:13316–13323
Marques JMC, Pais AACC, Abreu PE (2012) On the use of big-bang method to generate low-energy structures of atomic clusters modeled with pair potentials of different ranges. J Comput Chem 33:442–452
Lai XJ, Xu RC, Huang WQ (2011) Geometry optimization of bimetallic clusters using an efficient heuristic method. J Chem Phys 135:164109
Ye T, Xu RC, Huang WQ (2011) Global optimization of binary Lennard-Jones clusters using three perturbation operators. J Chem Inf Model 51:572–577
Calvo F, Yurtsever E (2004) Composition-induced structural transitions in mixed rare-gas clusters. Phys Rev B 70:045423
Frantz DD (1996) A computational study of 13-atom Ar–Kr cluster heat capacities. J Chem Phys 105:10030–10049
Doye JPK, Wales DJ, Berry RS (1995) The effect of the range of the potential on the structures of clusters. J Chem Phys 103:4234–4249
Doye JPK, Wales DJ (2001) Polytetrahedral clusters. Phys Rev Lett 86:5719–5722
Doye JPK (2003) A model metal potential exhibiting polytetrahedral clusters. J Chem Phys 119:1136–1147
Cerbelaud M, Ferrando R, Barcaro G, Fortunelli A (2011) Optimization of chemical ordering in AgAu nanoalloys. Phys Chem Chem Phys 13:10232–10240
Wu X, Wu YP, Kai XM, Wu GH, Chen YC (2011) Structural optimization of Ag–Pd clusters based on different potential parameterizations. Chem Phys 390:36–41
Acknowledgments
This study was supported by National Natural Science Foundation of China (NNSFC) (Nos. 21203002 and 21171008) and Anhui Provincial Natural Science Foundation (No. 1308085QB29). The authors thank X.G. Shao for a Grant from the Adaptive Immune Optimization Algorithm (AIOA) program from Nankai University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, X., Sun, Y., Gao, YC. et al. Structural transitions in mixed ternary noble gas clusters. J Mol Model 19, 3119–3125 (2013). https://doi.org/10.1007/s00894-013-1847-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-013-1847-3