Abstract
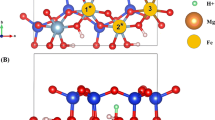
Density functional theory calculations were performed to investigate the adsorption and hydration of an ammonium ion (NH4 +) confined in the interlayer space of montmorillonites (MMT). NH4 + is trapped in the six-oxygen-ring on the internal surface and forms a strong binding with the surface O atoms. The hydration of NH4 + is affected significantly by the surface. Water molecules prefer the surface sites, and do not bind with the NH4 + unless enough water molecules are supplied. Moreover, the water molecules involved in NH4 + hydration tend to bind with the surface simultaneously. The hydration energy increases with the intercalated water molecules, in contrast to that in gas phase. In addition, the hydration leads to the extension of MMT basal spacing.

Hydrated ammonium ion inside montmorillonite







Similar content being viewed by others
References
Boek ES, Coveney PV, Skipper NT (1995) Monte Carlo molecular modeling studies of hydrated Li-, Na-, and K-smectites: understanding the role of potassium as a clay swelling inhibitor. J Am Chem Soc 117:12608–12617
Whitley HD, Smith DE (2004) Free energy, energy, and entropy of swelling in Cs-, Na-, and Sr-montmorillonite clays. J Chem Phys 120:5387–5395
Tunega D, Haberhauer G, Gerzabek MH, Lischka H (2002) Theoretical study of adsorption sites on the (001) surfaces of 1:1 clay minerals. Langmuir 18:139–147
Chatterjee A, Ebina T, Onodera Y, Mizukami F (2004) Effect of exchangeable cation on the swelling property of 2:1 dioctahedral smectite—A periodic first principle study. J Chem Phys 120:3414–3424
Liu XD, Lu XC (2006) A Thermodynamic understanding of clay-swelling inhibition by potassium ions. Angew Chem Int Ed 45:6300–6303
Tambach TJ, Bolhuis PG, Hensen EJM, Smit B (2006) Hysteresis in clay swelling induced by hydrogen bonding: accurate prediction of swelling states. Langmuir 22:1223–1234
Oueslati W, Karmous MS, Rhaiem HB, Lanson B, Amara ABH (2007) Effect of interlayer cation and relative humidity on the hydration properties of a dioctahedral smectite. Z Kristallogr Suppl 26:417–422
Morodome S, Kawamura K (2011) In situ X-ray diffraction study of the swelling of montmorillonite as affected by exchangeable cations and temperature. Clays Clay Miner 59:165–175
Zeng QH, Yu AB, Lu GQ, Standish RK (2003) Molecular dynamics simulation of organic–inorganic nanocomposites: layering behavior and interlayer structure of organoclays. Chem Mater 15:4732–4738
Liu XD, Lu XC, Meijer EJ, Wang RC (2012) Atomic-scale structures of interfaces between phyllosilicate edges and water. Geochim Cosmochim Acta 81:56–68
Gautier M, Muller F, Forestier LL, Beny JM, Guegan R (2010) NH4-smectite: characterization, hydration properties and hydro mechanical behaviour. Appl Clay Sci 49:247–254
Mingram B, Bräuer K (2001) Ammonium concentration and nitrogen isotope composition in metasedimentary rocks from different tectonometamorphic units of the European Variscan belt. Geochim Cosmochim Acta 65:273–287
Gieskes JM, Mahn C (2007) Halide systematics in interstitial waters of ocean drilling sediment cores. Appl Geochem 22:515–533
Christensen TH, Kjeldsen P, Bjerg PL, Jensen DL, Christensen JB, Baun A, Albrechtsen HJ, Heron C (2001) Biogeochemistry of landfill leachate plumes. Appl Geochem 16:659–718
Watenphul A, Wunder B, Heinrich W (2009) High-pressure ammonium-bearing silicates: implications for nitrogen and hydrogen storage in the Earth’s mantle. Am Mineral 94:283–292
Meziti C, Boukerroui A (2011) Regeneration of a solid waste from an edible oil refinery. Ceram Int 37:1953–1957
Achilias DS, Siafaka P, Nikolaidis AK (2012) Polymerization kinetics and thermal properties of poly(alkyl methacrylate)/organomodified montmorillonite nanocomposites. Polym Int 61:510–1518
Liu N, Wang MX, Liu MM, Liu F, Weng LP, Koopal LK, Tan WF (2012) Sorption of tetracycline on organo-montmorillonites. J Hazard Mater 225:28–35
Ma JZ, Deng FQ, Xue CH, Duan ZY (2012) Effect of modification of montmorillonite on the cellular structure and mechanical properties of ethylene vinyl acetate/clay nanocomposite foams. J Reinf Plast Compos 31:1170–1179
Mishra AK, Allauddin S, Narayan R, Aminabhavi TM, Raju KVSN (2012) Characterization of surface-modified montmorillonite nanocomposites. Ceram Int 38:929–934
Palkova H, Jankovic L, Zimowska M, Madejova J (2011) Alterations of the surface and morphology of tetraalkyl-ammonium modified montmorillonites upon acid treatment. J Colloid Interface Sci 363:213–222
Lin KJ, Jeng US, Lin KF (2011) Adsorption and intercalation processes of ionic surfactants on montmorillonite associated with their ionic charge. Mater Chem Phys 131:120–126
Tortora M, Gorrasi G, Vittoria V, Galli G, Ritrovati S, Chiellini E (2002) Structural characterization and transport properties of organically modified montmorillonite/polyurethane nanocomposites. Polymer 43:6147–6157
Drits VA, Lindgreen H, Salyn AL (1997) Determination of the content and distribution of fixed ammonium in illite-smectite by X-ray diffraction: Application to North Sea illite-smectite. Am Mineral 82:79–87
Bobos I, Ghergari L (1999) Conversion of smectite to ammonium illite in the hydrothermal system of Harghita Bặi, Romania: SEM and TEM investigations. Geol Carpath 50:379–387
Jankovic E, Komadel P (2000) Catalytic properties of a heated ammonium-saturated dioctahedral smectite. Collect Czechoslov Chem Commun 65:1527–1536
Bishop JL, Banin A, Mancinelli RL, Klovstad MR (2002) Detection of soluble and fixed NH4 + in clay minerals by DTA and IR reflectance spectroscopy: a potential tool for planetary surface exploration. Planet Space Sci 50:11–19
Pironon J, Pelletier M, de Donato P, Mosser-Ruck R (2003) Characterization of smectite and illite by FTIR spectroscopy of interlayer NH4 + cations. Clay Miner 38:201–211
Drits VA, Sakharov BA, Salyn AL, Lindgreen H (2005) Determination of the content and distribution of fixed ammonium in illite-smectite using a modified X-ray diffraction technique: application to oil source rocks of western Greenland. Am Mineral 90:71–84
Torsten S, Christian K (2009) Structural characterization of (Cu2+, Na+)- and (Cu2+, NH4 +)- exchanged bentonites upon thermal treatment. Clays Clay Miner 57:40–45
Shalabi AS, Assem MM, Soliman KA (2011) Adsorption and spin state properties of Cr, Ni, Mo, and Pt deposited on Li+ and Na+ monovalent cation impurities of MgO (001) surface: DFT calculations. J Mol Model 17:3299–3308
Boulet P, Knofel C, Kuchta B, Hornebecq V, Llewellyn PL (2012) Computational investigation of the adsorption of carbon dioxide onto zirconium oxide clusters. J Mol Model 18:4819–4830
Blowers P, Kim BG (2011) The adsorption of mercury-species on relaxed and rumpled CaO (0 0 1) surfaces investigated by density functional theory. J Mol Model 17:505–514
Lou ZY, Zeng Q, Chu X, Yang F, Yang ML, Xiang ML, Zhang XD, Fan HS (2012) First-principles study of the adsorption of lysine on hydroxyapatite (1 0 0) surface. Appl Surf Sci 258:4911–4916
Shi J, Liu HB, Lou ZY, Zhang Y, Meng YF, Zeng Q, Yang ML (2013) Effect of interlayer counterions on the structures of dry montmorillonites with Si4+/Al3+ substitution. Comput Mater 69:95–99
Zvyagin BB, Pinsker ZG (1949) Electron diffraction study of the montmorillonite structure. Dokl Acad Nauk SSSR 68:30–35
Tsipursky SI, Drits VD (1984) The distribution of octahedral cations in the 2:1 layers of dioctahedral smectites studied by oblique-texture electron diffraction. Clay Miner 19:177–193
Skipper NT, Chang FRC, Sposito G (1995) Monte Carlo simulation of interlayer molecular structure in swelling clay minerals. 1. Methodology. Clays Clay Miner 43:285–293
Viani A, Gualtieri AF, Artioli G (2002) The nature of disorder in montmorillonite by simulation of X-ray powder patterns. Am Mineral 87:966–975
Gournis D, Lappas A, Karakassides MA, Tobbens D, Moukarika A (2008) A neutron diffraction study of alkali cation migration in montmorillonites. Phys Chem Miner 35:49
Cygan RT, Liang JJ, Kalinichev AG (2004) Molecular models of hydroxide, oxyhydroxide, and clay phases and the development of a general force field. J Phys Chem B 108:1255–1266
Dvoyashkin M, Zang J, Yucelen GI, Katihar A, Nair S, Sholl DS, Bowers CR, Vasenkov S (2012) Diffusion of tetrafluoromethane in single-walled aluminosilicate nanotubes: pulsed field gradient NMR and molecular dynamics simulations. J Chem Phys C 116:21350–21355
Zhang GZ, Al-Saidi WA, Myshakin EM, Jordan KD (2012) Dispersion-corrected density functional theory and classical force field calculations of water loading on a pyrophyllite (001) surface. J Chem Phys C 116:17134–17141
Ledyastuti M, Liang YF, Kunieda M, Matsuoka T (2012) Asymmetric orientation of toluene molecules at oil-silica interfaces. J Chem Phys 137:064703–064711
Tokarsky J, Capkova P, Burda JV (2012) Structure and stability of kaolinite/TiO2 nanocomposite: DFT and MM computations. J Mol Model 18:2689–2698
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Sánchez-Portal D, Ordejón P, Artacho E, Soler JM (1997) Density-functional method for very large systems with LCAO basis sets. Int J Quantum Chem 65:453–461
Soler JM, Artacho E, Gale JD, García A, Junquera J, Ordejón P, Sáanchez-Portal D (2002) The Siesta method for ab initio order-N materials simulation. J Phys Condense Matter 14:2745–2779
Troullier N, Martins JL (1991) Efficient pseudopotentials for plane-wave calculations. Phys Rev B 43:1993–2006
Sainz-Díaz CI, Timón V, Botella V, Artacho E, Hernández-Laguna A (2002) Quantum mechanical calculations of dioctahedral 2:1 phyllosilicates: Effect of octahedral cation distributions in pyrophyllite, illite, and smectite. Am Mineral 87:958–965
Stich I, Parrinello M, Holender JM (1996) Dynamics, spin fluctuations, and bonding in liquid silicon. Phys Rev Lett 76:2077–2080
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27:1787–1799
Karaborni S, Smit B, Heidug W, Urai J, Oort EV (1996) The swelling of clays: molecular simulations of the hydration of montmorillonite. Science 271:1102–1104
Brugé F, Bernasconi M, Parrinello M (1999) Density-functional study of hydration of ammonium in water clusters. J Chem Phys 110:4734–4736
Acknowledgments
This work was supported by Open Research Fund of State Key Laboratory of Oil and Gas Reservoir Geology and Exploration (Southwest Petroleum University, No. PLN1118) and National Natural Science Foundation of China (No. 51134004 and 51204142). Part of computations was carried out on the Computer Clusters in Institute of Atomic and Molecular Physics, Sichuan University.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Shi, J., Liu, H., Meng, Y. et al. First-principles study of ammonium ions and their hydration in montmorillonites. J Mol Model 19, 1875–1881 (2013). https://doi.org/10.1007/s00894-012-1748-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1748-x