Abstract
A theoretical analysis of bacteriochlorophyll a containing its non-native divalent metal ions: Co, Ni, Cu, Zn, Ru, Rh, Pd, and Pt, has been carried out by means of density functional theory (DFT) calculations. The main stress was put on the derivatives with metals, which already found applications as coordination compounds in anti-tumor therapy (Ru, Pt, Pd, and Rh). The idea was to combine their cytotoxic properties with the known suitability of bacteriochlorophylls macrocycle for photodynamic therapy. The geometries of the studied systems are compared and reveal a number of similarities. The cores of the modified bacteriochlorophylls are flat, and the introduced metal ions lie in plane of the macrocycle, showing its large ability to accommodate metal ions of different sizes. However, four metal–nitrogen bonds, linking the central ions with the macrocycle ligand, are not equivalent. Metals are the strongest attached to nitrogens, which come from the pyrrole, which is fused with isocyclic ring. Based on the known spectroscopic data, the absorption properties of the proposed systems are predicted. Finally, it is found that all studied metal–macrocycle adducts are stable in aqueous media. The only exceptions are Mg-BChla (the finding is reflected by experimental facts) and Zn-BChla. The predicted high stability of Ru-, Rh-, Pt- and Pd-bacteriochlorophylls might turn out beneficial for therapeutic purposes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photodynamic therapy (PDT) is one of the methods to cure cancer, which uses the combination of a drug (called a photosensitizer) and a light [1–3]. Upon irradiation with the light of an appropriate wave-length, the photosensitizer is excited from its ground state (usually singlet) to the excited state - at first singlet, which may subsequently undergo conversion to triplet through inter-system crossing. The excess energy might then be transferred to nearby oxygen molecules and excite them from triplet ground state to singlet, thus producing highly reactive species, able to damage living cells. Otherwise, the excitation energy might be dissipated in a form of heat, causing cell damage through over-heating. At the end, the photosensitizer returns to its ground state.
The working principle of the photodynamic therapy implies several requirements, which should be fulfilled by good photosensitizers [2]. They should effectively absorb light in the range appropriate for medical applications, i.e., 620–850 nm, preferably red-shifted. Inversely, their absorption in the range 400–600 nm should be low in order to avoid prolonged skin sensitivity to the sunlight. Next, the efficiency of singlet to triplet intersystem crossing should be enhanced, which is usually achieved through incorporation of a metal ion into its structure. For the good candidates, it is sought that the singlet-triplet energy gap is higher than the energy needed to excite molecular oxygen (ca 1 eV). Last but not least, a high stability of these drugs is needed, both thermodynamic and photostability, as well as their low toxicity in the dark.
The application of porphyrin-based photosensitizers (porphyrins, texaphyrins, chlorins and bacteriochlorins) as agents for photodynamic therapy has draw attention in the last couple of years due to their good light-absorbing properties.
Synthetic porphyrins, hematoporphyrins and their analogues served as first photosensitizers, but they showed a number of drawbacks [4], among which were high retention times in living organisms, causing prolonged skin sensitivity to light and relatively low absorption, resulting in the need of the increased doses. Nowadays the researchers focus rather on the naturally occurring porphyrins, e.g., chlorophylls and bacteriochlorophylls [5, 6], as these have shorter retention times and their decomposition pathways are already established. Among different derivatives of porphyrins, bacteriochlorophylls offer remarkable properties, which are mainly due to the fact that their absorption spectrum is considerably red-shifted.
Therefore the current research concentrates on modifications of structure and properties of the native bacteriochlorophylls (and chlorophylls), which might be important for medical applications, and allow to over-come known drawbacks of synthetic photosensitizers.
The replacement of the central magnesium ion for a heavy element ameliorates photophysical behavior of these pigments by enhancing singlet-triplet intersystem crossing due to spin-orbit coupling [7]. Another modification may cover the existing side chains, substituting the macrocycle core. For instance, it is shown that the lack of phytyl (a C20 alcohol moiety attached to chlorophylls and bacteriochlorophylls) ameliorates their solubility in aqueous media [8].
On the other hand, a number of transition metal ions in form of their complexes found application in anti-tumor therapy [9]. Platinum(II) complexes are the parent drugs, as they are used for chemotherapy against, e.g., lung and bladder cancers [10]. The second widely known transition metal in this area is ruthenium. In this context, usually Ru(III) salts are studied [11], however, it is believed that in organisms Ru(III) undergoes reduction to Ru(II) with ascorbic acid or other reductants [12, 13]. Among others, Pd(II) and Rh(II) are also popular and offer new possibilities for treating new types of cancer cells [9].
It seems to be desirable that these two aspects of metal-containing compounds are combined in a single molecule, which may act in two different ways to fight cancer cells. Therefore, the idea to use Pt, Ru, Pd, and Rh substituted bacteriochlorophylls appears tempting. On the one hand, the absorption properties of bacteriochlorophylls would be used in standard PDT, while on the other hand the anticancer properties of the metal ions will be employed after the treatment with light.
Consequently, the aim of the present study is to characterize different metallo-substituted bacteriochlorophylls, as possible candidates for photodynamic therapy. Special attention is paid to derivatives, which contain transition metal ions, whose anti-tumor activity is known and new systems, in which they may render useful, are sought. Additionally, the set of tested metals is enlarged by Mg (a native metal in bacteriochlorophyll), Co, Ni, Cu, Zn. The selection of the latter follows from the availability of spectroscopic characterization of their bacteriochlorophyll derivatives [6], made in relation to the possible application of these species in photodynamic therapy.
Studies of similar nature might be found in literature. The most extensive studies of different possible photosensitizers – derivatives of porphyrin and texaphyrin with and without central magnesium atom for use in PDT are published by Russo and his co-workers [14–16]. Additionally, the selected adducts of bacteriochlorins with metals of the first transition row are characterized and their spectral properties are discussed in detail [17]. The main stress was put on the characterization of their absorption spectra, which were rationalized with an aid of time-dependent density functional theory.
The present research is focused on the adducts with metals, which already found application in anti-cancer therapy. In this respect our study covers a different range of tested metal ions than in Russo’s studies. Moreover, the macrocyclic ligand, which is used throughout the present study, contains the naturally occurring bacteriochlorin ring substituents, thus they may be synthetized on the base of natural materials.
One should mention that the theoretical studies of the spectroscopic properties of the native chlorophylls and bacteriochlorophylls are also published (e.g., [18–21]), but they were undertaken rather in order to elucidate the details of the light absorption in photosynthesis, and not in view of their potential applications in PDT. An interested reader should be referred to the extensive reviews [20, 22, 23] and references therein.
Methods and model
In the present study quantum chemical method based on density functional theory (DFT) with non-local Becke-Perdew functional was applied [24–28]. The calculation consisted of full geometry optimizations of the studied structures and was further confirmed with vibrational analysis. In order to accelerate computation the resolution-of-identity (RI) algorithm was applied [29, 30]. All-electron Gaussian type orbitals of def2-TZVP quality were used to define atomic orbitals [31], while effective core potentials were applied for heavy elements (Ru, Rh, Pd, and Pt). The solvation was accounted for by COSMO model [32] with default radii for the elements (H = 1.30, C = 2.00, N = 1.83, O = 1.72) and 2.00 Å for the studied metal ions. Dielectric permittivity ε equal to 80 was used in order to take into account the nature of the possible aqueous environment in which these potential therapeutic agents will appear in tissues. The present results were obtained with Turbomole v. 6.3 [33] and further analyzed with AOMix 6.6 program package [34, 35].
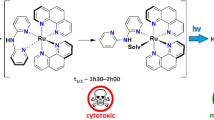
The theoretical model of the studied system is shown in Fig. 1. It consists of the bacteriochlorophyll a molecule bearing all its native substituents, except the long phytyl chain, which is here replaced by a hydrogen atom (so called a bacteriopheophorbide ligand). Such a simplification of the model is justified in light of the fact that the lack of phytyl ameliorates solubility of bacteriochlorophyll in aqueous media and is beneficial for its prospect medical application.
In the investigated bacteriochlorophyll molecule four pyrroles of the macrocycle are not equivalent, so the symmetry of the molecule is lowered to C1 as compared to C4V in porphyrins or D2h as in bacteriochlorins. This is due to the presence of different substituents in the macrocyle periphery and to the fact that two of four pyrroles are saturated, while the remaining two are not.
In the present paper eight bacteriochlorophyll derivatives are studied, in which a native central metal ion – magnesium - is replaced for another metal. The studied metal ions cover: Co(II), Ni(II), Cu(II), Zn(II), Ru(II), Rh(II), Pd(II), and Pt(II). For all of the metals + II oxidation state is common and ensures the neutral charge of the whole system. As one may notice, the range of the studied central metal ions is wider than those of anti-cancer activity, outlined in the introduction. This is a result of the availability of spectroscopic data [6], which due to structure-activity relationship models may be compared with computed parameters and help to foresee the properties of derivatives not characterized thus far. Such an approach already proved useful in forecasting the physico-chemical characteristics of different groups of molecules [36, 37].
Results and discussion
In the following, the description of the investigated bacteriochlorophyll derivatives is given, with an emphasis on their structural and electronic properties. The discussion is then shifted to the problem of the stability of the chosen metal ions in bacteriochlorophyll cavity in aqueous environment.
Characteristics of bacteriochlorophylls containing different central metals
The basic structural parameters obtained throughout the present study are listed in Table 1. In all but one of the investigated cases, the ground state of the molecules is low spin, which is singlet or dublet, depending on the type of the central metal ion. The only odd system is Ru-BChla, whose triplet lies the lowest on the potential energy surface: singlet and pentet states are higher by 0.39 eV and 1.20 eV, respectively. These findings are in agreement with previous theoretical study, in which different transition metal ions from the fourth period have been examined [17]. While most of the studied systems exhibited low spin, iron-bacteriochlorin was characterized by the intermediate spin. The similarity of the ground state multiplicity is a consequence of the location of the two elements in the periodic table: ruthenium lies directly below iron.
The singlet-triplet energy separation is an important parameter regarding proposed application of the investigated systems for photodynamic therapy. The energy gap should be higher than required to excite molecular oxygen to its singlet state. In this respect both new bacteriochlorophyll derivatives, whose ground state is singlet (with Pt and Pd), turn up suitable. The singlet–triplet energy separation is 1.15 eV and 1.11 eV for Pt-BChla and Pd-BChla, respectively.
The analysis of the geometries of M-BChla systems reveal that all cores of the macrocyclic complexes are planar, independent of the type of the central metal ion. This clearly shows the flexibility of the bacteriochlorin macrocycle and its ability to accommodate ions of different radii. Out-of-plane displacement of the central metals in porphyrin-related systems, often reported in literature, is related strictly to the presence of additional ligands, which pull them from the center of the macrocycle [38–40].
Four pyrroles, which constitute the core of bacteriochlorin, are not equivalent and so four metal–nitrogen bonds are not of the same length. In all studied systems one may observe a similar pattern: the shortest bond is formed with the nitrogen from the pyrrole C, which is fused with the isocyclic ring, while the longest with the nitrogen to which originally phytyl is attached (pyrrole D). The remaining two bonds are neither of the same length: the shorter connects metal with nitrogen of pyrrole A, while the longer with nitrogen from pyrrole B.
One may then measure the size of the central cavity, which has a shorter diameter along the line connecting pyrroles A and C, and a larger diameter connecting pyrroles B and D; the difference between them may reach up to 9 %, as in Zn-BChla. The macrocycle core is the widest in Zn-BChla (two diameters are equal to 3.969 Å and 4.327 Å), and the tightest in Ni-BChla (3.874 Å and 3.998 Å).
One should notice then that the central metal is never located in the exact center of the cavity, but is always shifted toward the fragment of the macrocycle of a larger electron density (pyrrole C fused with the isocyclic ring) [41]. This feature is also reported by Sundholm [21] in his theoretical studies of bacteriochlorophyll b species. It is thus due to the presence of macrocycle substituents and the isocyclic ring. The non-equivalency of four metal–nitrogen bonds is further reflected by their bond orders. It should be born in mind, however, that in real conditions the nuclear position will not be static due to thermal vibrations. All nuclei will be able to occupy a certain volume around the determined point in space, and their average location will be determined by experiment.
Next, the energies of frontier orbitals are compared. HOMO levels are similar and vary from -4.95 eV for Rh-BChla to -4.83 eV for Mg-BChla, while LUMO levels vary from -3.73 eV for Cu-BChla to -3.58 eV for Ru-BChla. Consequently, the HOMO-LUMO gaps of all the studied derivatives are similar and fall in the range 1.15–1.35 eV. Such small differences arise from the fact that frontier orbitals are located mostly on the macrocycle, with small contributions from metal orbitals appearing only in LUMOs – see Supplementary material. This finding is in line with the results obtained for model metallobacteriochlorins, where similar character of HOMOs and LUMOs was observed [17]. The bacteriochlorophyll derivatives covered in this study have additional isocyclic ring, therefore their frontier orbitals contain also its admixture. This gives rise to the before-mentioned shifted position of central metal ions too.
The UV–VIS spectra of a majority of the studied compounds can be found in literature [6]. The analysis of the absorption properties reveals their potential applicability for photodynamic therapy. The Qx band is red-shifted, ensuring the use of red light for irradiation, which penetrates deeper into tissues than other wavelengths form the visible spectrum.
According to our knowledge, only some of the metallobacteriochlorophylls described here are characterized in literature. An attempt is then made to somehow predict the absorption edge based on the calculated properties of the remaining species. In order to do so the experimental peak positions of the adsorption band (which in the literature are related to as Qx band) are then correlated with the HOMO-LUMO gap. The obtained results show a linear relationship between the two values with the correlation coefficient equal to 0.89. The plot of the regression is shown in Fig. 2. The calculated parameters of the regression line serve to approximate the adsorption band of the bacteriochlorophyll derivatives whose spectra are not published. Therefore it is predicted that Pt-BChla would absorb at 752 nm, Ru-BChla at 738 nm, and Rh-BChla at 745 nm. These values confirm the applicability of the abovementioned species to be used as light absorbing agents in PDT.
Central metal leaching
As was already underlined, one of the main aspects during the test process of new therapeutic agents is their stability and toxicity. In the case of pharmaceuticals containing transition metal ions one should consider the possibility of the central metal leaching into the body fluids. While some of the metal ions are neutral or even beneficial for human health in moderate concentrations (here, e.g., magnesium), some are toxic and thus may have detrimental effect on the functioning of the body (here, e.g., cobalt or platinum). To somehow assess a possibility of leaching of the central metal, the exchange of central metals for protons from environment is considered as described by the following reaction:
It is assumed that two protons, which are initially solvated (each one with two water molecules), replace the central metal ion, which forms an aqua complex afterward. The number of aqua ligands is typical for a given metal ion and equals six for Mg, Co, Cu, Ru, Rh and four for Zn, Pt, Pd, Ni.
The idea to relate the metallobacteriochlorophyll stability to the resistance toward the displacement of the central metal by acid is generally accepted in metalloporphyrin research [42–44]. This is because 1) it is often difficult to determine equilibrium constants directly and 2) metalloporphyrins show a wide range of sensitivity to acid.
It is then proposed that the measure of the stability of the metal-ligand adduct is the energetic cost of the abovementioned process, calculated in aqueous phase (accounted for with COSMO model):
One should emphasize here, that the stability, as defined in this paper, is related only to the thermodynamic property and does not discuss the kinetic stability of the system. The latter would be connected with the height of energy barrier which should be overcome to reach the products.
The computed values are listed in Table 1. The analysis of the collected data reveals that the native central atom of bacteriochlorophyll, magnesium, is not stable in the macrocycle cavity. This observation is not surprising, as the low stability of magnesium porphyrins, chlorins and bacteriochlorins is a well-known fact [8, 45, 46], further reflected by the high cost of these synthetic materials.
The stabilization of the zinc derivative is minor. The energy needed for Zn2+ to be replaced by two protons is lower that 1 kcal mol−1. This means that the application of Zn-BChla into the tissues might result in the decomposition of the complex into bacteriochlorophyll and the hydrated zinc ion provided the Zn-BChla complex is labile.
The other metals are stable while inserted into bacteriochlorophyll cavity, with metal exchange energies being larger than 15 kcal mol−1. The calculated energy exchange allows to rank them in line with the increased predicted stability: Cu < Co < Ru < Ni < Rh < Pd < Pt.
Traditionally, the stability of metal-substituted porphyrins is defined by so-called “stability index” which is proportional to Pauling electronegativity of an element and inversely proportional to its effective ionic radius [44]. Although both scales do not agree perfectly, the general trend is conserved. Mg derivative is characterized by the lowest stability index and Zn-BChla is the second least stable, the same as predicted in the present paper. Platinum and palladium derivatives show high stability, while copper and cobalt are in the range of medium values. The largest discrepancy is found for nickel adduct; it is characterized by the highest stability index, while its exchange energy places it below Pd and Pt complexes.
In view of the potential applications of the studied metal-bacteriochlorins for therapeutic purposes, the high stability of Pt, Pd, Rh and Ru derivatives might be beneficial. These metal ions are cytotoxic and thus their uncontrolled release in tissues might result in more harm than is gained from their therapeutic properties. On the other hand, if there is a way to release them selectively in tumor cells, one may profit from their anti-cancer activity in situ. Indeed, one may imagine that after they serve as photosensitizers in PDT, the large macrocyclic ligand might undergo decomposition, e.g., upon irradiation or induced by endogenous or exogenous factors. As a result, the therapeutic metal ions would be released from bacteriochlorophyll “wrap” and present in the exact place for their action through mechanisms, which are proposed for their “standard” complexes. This type of action, which would profit from these two aspects of metallobacteriochlorophylls, might appear advantageous. On the one hand the photophysical properties of the macrocyclic fragment would be in use during light irradiation phase and on the second hand, their central metals might serve as therapeutic tools to fight with anomalous cells afterward.
Conclusions
The present study allowed for the structural characterization of bacteriochlorophylls containing non-native divalent metal ions: Co, Ni, Cu, Zn, Ru, Rh, Pd, and Pt. The main stress was put on the derivatives with metals, which already found applications in anti-tumor therapy in the form of other complexes, with an aim to combine their properties with the known suitability of bacteriochlorophylls to photodynamic therapy. The performed characterization of a wider group of species allowed for the prediction of unknown properties of these promising agents.
The geometry structures of the studied species show the surprising flexibility of bacteriochlorophyll cavity, which manifests itself in an ability to accommodate metal ions of different sizes.
The investigated species were also checked in view of their stability in water. The test reaction, which was the exchange of the central metal ion for two protons showed that all studied metal–macrocycle adducts are stable in aqueous media, except for Mg-BChla. The stability of Zn-BChla would be minor and in real conditions would depend mostly on the kinetics of its decomposition. The predicted inertness of the synthetic derivatives with metals of anti-tumor activity (Ru, Pt, Pd, Rh) might turn out beneficial for therapeutic purposes. They are expected not to decompose during the photodynamic therapy, while one may hopefully control their decomposition in the second phase of a therapy.
References
Institute NC. http://www.cancer.gov.
Szaciłowski K, Macyk W, Drzewiecka-Matuszek A, Brindell M, Stochel G (2005) Bioinorganic photochemistry: frontiers and mechanisms. Chem Rev 105:2647–2694
Bonnett R (2003) Metal complexes for photodynamic therapy. In: McCleverty JA, Meyer TJ (eds) Comprehensive coordination chemistry. Elsevier, Amsterdam
Jori G (1996) Tumour photosensitizers: approaches to enhance the selectivity and efficiency of photodynamic therapy. J Photochem Photobiol B: Biol 36:87–93
Rosenbach-Belkin VC L, Fiedor L, Salomon Y, Scherz A (1998) Chlorophyll and bacteriochlorophyll derivatives as photodynamic agents. In: Moser JG (ed) Photodynamic tumor therapy, 2nd and 3rd generation photosensitizers. Harwood Academic, Amsterdam, pp 117–125
Hartwich G, Fiedor L, Simonin I, Cmiel E, Schäfer W, Noy D, Scherz A, Scheer H (1998) Metal-substituted bacteriochlorophylls. 1. Preparation and influence of metal and coordination on spectra. J Am Chem Soc 120(15):3675–3683. doi:10.1021/ja970874u
Drzewiecka-Matuszek A, Skalna A, Karocki A, Stochel G, Fiedor L (2005) J Biol Inorg Chem 10:453–462
Scheer H (1991) Chlorophylls. CRC, Boca Raton
Berg SJLJM (1994) Principles of bioinorganic chemistry. University Science Books, Herndon, VA
Wheate NJ, Walker S, Craig GE, Oun R (2010) The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans 39(35):8113–8127
Mazuryk O, Kurpiewska K, Lewinski K, Stochel G, Brindell M (2012) Interaction of apo-transferrin with anticancer ruthenium complexes NAMI-A and its reduced form. J Inorg Biochem 116C:11–18. doi:10.1016/j.jinorgbio.2012.07.017
Brindell M, Elmroth SK, Stochel G (2004) Mechanistic information on the reaction of cis- and trans-[RuCl2(DMSO)4] with d(T2GGT2) derived from MALDI-TOF and HPLC studies. J Inorg Biochem 98(8):1367–1377. doi:10.1016/j.jinorgbio.2004.04.015
Brindell M, Stawoska I, Supel J, Skoczowski A, Stochel G, van Eldik R (2008) The reduction of (ImH)[trans-RuIIICl4(dmso)(Im)] under physiological conditions: preferential reaction of the reduced complex with human serum albumin. J Biol Inorg Chem 13(6):909–918. doi:10.1007/s00775-008-0378-3
Petit L, Quartarolo A, Adamo C, Russo N (2006) Spectroscopic properties of porphyrin-like photosensitizers:‚Äâ insights from theory. J Phys Chem B 110(5):2398–2404. doi:10.1021/jp055016w
Lanzo I, Russo N, Sicilia E (2008) First-principle time-dependent study of magnesium-containing porphyrin-like compounds potentially useful for their application in photodynamic therapy. J Phys Chem B 112(13):4123–4130. doi:10.1021/jp710880x
Quartarolo A, Russo N, Sicilia E, Lelj F (2007) Absorption spectra of the potential photodynamic therapy photosensitizers texaphyrins complexes: a theoretical analysis. J Comput Theor Chem 3(3):860–869
Petit L, Adamo C, Russo N (2005) Absorption spectra of first-row transition metal complexes of bacteriochlorins: a theoretical analysis. J Phys Chem B 109(24):12214–12221. doi:10.1021/jp050667d
Sundholm D (1999) Density functional theory calculations of the visible spectrum of chlorophyll a. Chem Phys Lett 302(5–6):480–484. doi:10.1016/s0009-2614(99)00194-3
Sundholm D (2000) Comparison of the electronic excitation spectra of chlorophyll a and pheophytin a calculated at density functional theory level. Chem Phys Lett 317(6):545–552. doi:10.1016/s0009-2614(99)01428-1
Ellervee A, Linnanto J, Freiberg A (2004) Spectroscopic and quantum chemical study of pressure effects on solvated chlorophyll. Chem Phys Lett 394(1):80–84. doi:10.1016/j.cplett.2004.06.113
Sundholm D (2003) A density-functional-theory study of bacteriochlorophyll b. Phys Chem Chem Phys 5(19):4265–4271
Linnanto J, Korppi-Tommola J (2006) Quantum chemical simulation of excited states of chlorophylls, bacteriochlorophylls and their complexes. Phys Chem Chem Phys 8(6):663–687
Alberto ME, Marino T, Russo N, Sicilia E, Toscano M (2012) The performance of density functional based methods in the description of selected biological systems and processes. Phys Chem Chem Phys 14(43):14943–14953. doi:10.1039/c2cp41836c
Dirac PAM (1929) Quantum mechanics of many-electron systems. Proc Royal Society of London Series A 123(792):714–733. doi:10.1098/rspa.1929.0094
Slater JC (1951) A simplification of the Hartree-Fock method. Phys Rev 81(3):385–390
Vosko SH, Wilk L, Nusair M (1980) Accurate spin-dependent electron liquid correlation energies for local spin density calculations: a critical analysis. Can J Phys 58(8):1200–1211. doi:10.1139/p80-159
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38(6):3098–3100
Perdew JP (1986) Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys Rev B 33(12):8822–8824
Eichkorn K, Treutler O, Öhm H, Häser M, Ahlrichs R (1995) Auxiliary basis sets to approximate coulomb potentials. Chem Phys Lett 240:283–289
Eichkorn K, Weigend F, Treutler O, Ahlrichs R (1997) Theoretical Chemistry Accounts 97:119–124
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys Chem Chem Phys 7(18):3297–3305
Klamt A, Schuurmann G (1993) COSMO: a new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J Chem Soc, Perkin Trans 2(5):799–805
TURBOMOLE V6.3 2011 adoUoKa, Forschungszentrum Karlsruhe GmbH -, TURBOMOLE GmbH saf, http://www.turbomole.com.
S. I. Gorelsky APfMOA, http://www.sg-chem.net/, University of Ottawa, version 6.5, 2011.
Gorelsky SI, Lever ABP (2001) Electronic structure and spectra of ruthenium diimine complexes by density functional theory and INDO/S. Comparison of the two methods. J Organomet Chem 635:187–196
Szaleniec M, Ryszard T, Witko M (2008) How to select an optimal neural model of chemical reactivity? Neurocomputing 72(1-3):241–256
Szaleniec M, Witko M, Heider J (2008) Quantum chemical modelling of the C–H cleavage mechanism in oxidation of ethylbenzene and its derivates by ethylbenzene dehydrogenase. J MolCataly A: Chem 286(1-2):128–136
Rutkowska-Zbik D, Korona T (2012) How many ligands can be bound by magnesium-porphyrin - a symmetry-adapted perturbation theory study. J Chem Theory Comput 8(8):2972–2982. doi:10.1021/ct300281p
Szaleniec M, Tokarz-Sobieraj R, Witko M (2008) Modification of tetraphenylporphyrin ring in manganese(III) complexes - theoretical description of the influence on their physicochemical properties. Pol J Chem 82:1853–1863
Timkovich R, Tulinsky A (1969) Structure of aquomagnesium tetraphenylporphyrin. J Am Chem Soc 91(16):4430–4432. doi:10.1021/ja01044a018
Orzel L, Kania A, Rutkowska-Zbik D, Susz A, Stochel G, Fiedor L (2010) Structural and electronic effects in the metalation of porphyrinoids. Theory and experiment. Inorg Chem 49(16):7362–7371. doi:10.1021/ic100466s
Lavallee DK (1985) Kinetics and mechanisms of metalloporphyrin reactions. Coord Chem Rev 61:55–96
Hambright P (2000) Chemistry of Water Soluble Porphyrins. In: Kadish KM, Smith KM, Guilard R (eds) The porphyrin handbook; Inorganic, organometallic and coordination chemistry, vol 3. Academic, San Diego, pp 130–210
Buchler JW (1975) In: Smith KC (ed) Porphyrins and metalloporphyrins. Elsevier, Amsterdam, p 157
Orzeł Ł, van Eldik R, Fiedor L, Gy S (2009) Mechanistic information on CuII metalation and transmetalation of chlorophylls. Eur J Inorg Chem 2009(16):2393–2406. doi:10.1002/ejic.200800662
Brindell M, Stawoska I, Orzeł L, Labuz P, Stochel G, van Eldik R (2008) Application of high pressure laser flash photolysis in studies on selected hemoprotein reactions. Biochim Biophys Acta (BBA) - Proteins Proteomics 1784(11):1481–1492. doi:10.1016/j.bbapap.2008.08.006
Acknowledgments
The work was supported by the Ministry of Science and Higher Education within the project no N N204 439640 in years 2011–2014.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 774 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Rutkowska-Zbik, D., Witko, M. Metallobacteriochlorophylls as potential dual agents for photodynamic therapy and chemotherapy. J Mol Model 19, 4155–4161 (2013). https://doi.org/10.1007/s00894-012-1747-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1747-y