Abstract
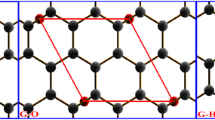
To further understand the structure of graphene oxide, several structures of graphene oxide were systematically investigated using density functional theory (DFT). Our models consisted of a hexagonal in-plane structure of graphene with epoxy groups, and different oxidation levels. We found that different arrangements of these units yielded a range of vibrational spectra. Raman positions of the D and G bands depend sensitively on the local atomic configurations. Both structure energy and spectra computations indicate that the oxidation functional groups are energetically favorable to aggregate together and to be close to one another on the opposite side of graphene surface.




Similar content being viewed by others
References
Liu F, Ming P, Li J (2007) Phys Rev B 76:064120.1–064120.7
Frank IW, Tanenbaum DM, van der Zande AM, McEuen PL (2007) J Vac Sci Tech B 25:2558–2561
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Science 306:666–669
Nair RR, Blake P, Grigorenko AN, Novoselov KS, Booth TJ, Stauber T, Peres NMR, Geim AK (2008) Science 320:1308–1308
Wang F, Zhang YB, Tian CS, Girit C, Zettl A, Crommie M, Shen YR (2008) Science 320:206–209
Ghosh S, Calizo I, Teweldebrhan D, Pokatilov EP, Nika DL, Balandin AA, Bao W, Miao F, Lau CN (2008) Appl Phys Lett 92:151911.1–151911.3
Balandin AA, Ghosh S, Bao WZ, Calizo I, Teweldebrhan D, Miao F, Lau CN (2008) Nano Lett 8:902–907
Geim AK, Novoselov KS (2007) Nat Mat 6:183–191
Landau LD (1937) Phys Z Sowjetunion 11:26–35
Peierls R (1935) Ann Inst Henri Poincare 5:177–182
Lahaye RJWE, Jeong HK, Park CY, Lee YH (2009) Phys Rev B 79:125435.1–125435.8
Dinh LD, Gunn K, Kyung JH, Young HL (2010) Phys Chem Chem Phys 12:1595–1599
Fuente E, Menéndez JA, Díez MA, Suárez D, Montes-Morán MA (2003) J Phys Chem B 107:6350–6359
Kudin KN, Ozbas B, Schniepp HC, Prud’homme RK, Aksay IA, Car R (2008) Nano Lett 8:36–41
Li ZY, Zhang WH, Luo Y, Yang JL, Hou JG (2009) J Am Chem Soc 131:6320–6321
Pandey D, Reifenberger R, Piner R (2008) Surf Sci 602:1607–1613
Li JL, Kudin KN, McAllister MJ, Prud’homme RK, Aksay IA, Car R (2006) Phys Rev Lett 96:176101.1–176101.4
Hofmann U, Holst R (1939) Ber Dtsch Chem Ges 72:754–771
Buchsteiner A, Lerf A, Pieper J (2006) J Phys Chem B 110:22328–22338
Boukhvalov DW, Katsnelson MI (2008) J Am Chem Soc 130:10697–10701
Segall MD, Lindan PLD, Probert MJ, Pickard CJ, Hasnip PJ, Clark SJ, Payne MC (2002) J Phys Condens Matter 14:2717–2744
Vosko SJ, Wilk L, Nusair M (1980) Can J Phys 58:1200–1211
Ceperley DM, Alder BJ (1980) Phys Rev Lett 45:566–569
Perdew JP, Zunger A (1981) Phys Rev B 23:5048–5079
Ooi N, Rairkar A, Adams JB (2006) Carbon 44:231–242
Qin W, Li X, Bian WW, Fan XJ, Qi JY (2010) Biomaterials 31:1007–1016
Yan JA, Ruan WY, Chou MY (2008) Phys Rev B 77:125401.1–125401.7
Yan JA, Xian L, Chou MY (2009) Phys Rev Lett 103:086802.1–086802.4
Xu Z, Xue K (2010) Nanotechnology 21:045704–045707
Hernández Rosas JJ, Ramírez Gutiérrez RE, Escobedo-Morales A, Chigo Anota E (2011) J Mol Model 17:1133–1139
Hamann DR, Schlüter M, Chiang C (1979) Phys Rev Lett 43:1494–1497
Monkhorst HJ, Pack JD (1976) Phys Rev B 13:5188–5192
Fischer TH, Almlof J (1992) J Phys Chem 96:9768–9774
He H, Klinowski J, Forster M, Lerf A (1998) Chem Phys Lett 287:53–56
Lerf A, He H, Forster M, Klinowski J (1998) J Phys Chem B 102:4477–4482
Cai W, Piner RD, Stadermann FJ, Park S, Shaibat MA, Ishii Y, Yang D, Velamakanni A, An SJ, Stoller M, An J, Chen D, Ruoff RS (2008) Science 321:1815–1817
Yan JA, Chou MY (2010) Phys Rev B 82:125403.1–125403.10
Stankovich S, Dikin DA, Piner R, Kohlhaas KM, Kleinhammes A, Jia Y, Wu Y, Nguyen ST, Ruoff RS (2007) Carbon 45:1558–1565
Lambert TN, Luhrs CC, Chavez CA, Wakeland S, Brumbach MT, Alam TM (2010) Carbon 48:4081–4089
Ferrari AC, Robertson J (2000) Phys Rev B 61:14095–14107
Akhavan O (2010) Carbon 48:509–519
Zhu Y, Murali S, Cai W, Li X, Suk JW, Potts JR, Ruoff RS (2010) Adv Mater 22:3906–3924
Ferrari AC, Meyer JC, Scardaci V, Casiraghi C, Lazzeri M, Mauri F, Piscanec S, Jiang D, Novoselov KS, Roth S, Geim AK (2006) Phys Rev Lett 97:187401.1–187401.4
Graf D, Molitor F, Ensslin K, Stampfer C, Jungen A, Hierold C, Wirtz L (2007) Nano Lett 7:238–242
Bulat FA, Burgess JS, Matis BR, Baldwin JW, Macaveiu L, Murray JS, Politzer P (2012) J Phys Chem A 116:8644–8652
Acknowledgments
This research used computational resources at the China Academy of Engineering Physics. This work is supported by the National Natural Science Foundation of China (Grant No. 41272051) and the Postgraduate Innovation Fund sponsored by Southwest University of Science and Technology (Grant No.12ycjj22). It is also supported by the Doctoral Fund of Southwest University of Science and Technology (Grant No.11ZX7135).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, B., Sun, H., Peng, T. et al. Molecular vibrational spectroscopy characterization of epoxy graphene oxide from density functional calculations. J Mol Model 19, 1429–1434 (2013). https://doi.org/10.1007/s00894-012-1701-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1701-z