Abstract
A combined and sequential use of quantum mechanical (QM) calculations and classical molecular dynamics (MD) simulations was made to investigate the σ and π types of hydrogen bond (HB) in benzene-water and pyrrole-water as clusters and as their liquid mixture, respectively. This paper aims at analyzing similarities and differences of these HBs resulted from QM and MD on an equal footing. Based on the optimized geometry at ωb97xD/aug-cc-pVTZ level of theory, the nature and property of σ and π types of HBs are unveiled by means of atoms in molecules (AIM), natural bond orbital (NBO) and energy decomposition analysis (EDA). In light of the above findings, MD simulation with OPLS-AA and SPC model was applied to study the liquid mixture at different temperatures. The MD results further characterize the behavior and structural properties of σ and π types HBs, which are somewhat different but reasonable for the clusters by QM. Finally, we provide a reasonable explanation for the different solubility between benzene/water and pyrrole/water.

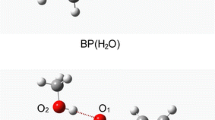
The σ and π types of hydrogen bond as benzene-water and pyrrole-water clusters in gas; Snapshot of benzene/water and pyrrole/water as 1:1 liquid mixture extracted from the MD simulations






Similar content being viewed by others
References
Mata I, Alkorta I, Molins E, Espinosa E (2010) Chem Eur J 16:2442–2452
Tsuzuki S, Uchimaru T (2006) Curr Org Chem 10:745–762
Scheiner S, Kar T, Pattanayak J (2002) J Am Chem Soc 124:13257–13264
Riley KE, Pitoňák M, Černý J, Hobza P (2010) J Chem Theory Comput 6:66–80
Donoso-Tauda O, Jaque P, Santos JC (2011) Phys Chem Chem Phys 13:1552–1559
Tsuzuki S, Fujii A (2008) Phys Chem Chem Phys 10:2584–2594
Tsuzuki S, Honda K, Uchimaru T, Mikami M, Tanabe K (2000) J Am Chem Soc 122:11450–11458
Meyer EA, Castellano RK, Diederich F (2003) Angew Chem Int Ed 42:1210–1250
Hobza P, Müller-Dethlefs K (2010) Non-covalent interactions: theory and experiment. RSC, Cambridge
Carles S, Lecomte F, Schermann JP, Desfrançáois C (2000) J Phys Chem A 104:10662–10668
Maris A, Melandri S, Miazzi M, Zerbetto F (2008) ChemPhysChem 9:1303–1308
Crittenden DL (2009) J Phys Chem A 113:1663–1669
Mishra BK, Sathyamurthy N (2007) J Phys Chem A 111:2139–2147
Schwartz CP, Uejio JS, Duffin AM, England AH, Prendergast D, Saykally RJ (2009) J Chem Phys 131:114509
Nagy PI, Durant GJ, Smith DA (1993) J Am Chem Soc 115:2912–2922
Janjić GV, Veljković DŽ, Zarić SD (2011) Cryst Growth Des 11:2680–2683
Suzuki S, Green PG, Bumgarner RE, Dasgupta S, Goddard WA III, Blake GA (1992) Science 257:942–945
Augspurger JD, Dykstra CE, Zwier TS (1993) J Phys Chem 97:980–984
Tarakeshwar P, Choi HS, Lee SJ, Lee JY, Kim KS, Ha T-K, Jang JH, Lee JG, Lee H (1999) J Chem Phys 111:5838–5850
Prakash M, Samy KG, Subramanian V (2009) J Phys Chem A 113:13845–13852
Feller D (1999) J Phys Chem A 103:7558–7561
Ma J, Alfè D, Michaelides A, Wang E (2009) J Chem Phys 130:154303
Li S, Cooper VR, Thonhauser T, Puzder A, Langreth DC (2008) J Phys Chem A 112:9031–9036
Slipchenko LV, Gordon MS (2009) J Phys Chem A 113:2092–2102
Gierszal KP, Davis JG, Hands MD, Wilcox DS, Slipchenko LV, Ben-Amotz DJ (2011) J Phys Chem Lett 2:2930–2933
Raschke TM, Levitt M (2004) J Phys Chem B 108:13492–13500
Keresztúri Á, Jedlovszky P (2005) J Phys Chem B 109:16782–16793
Ikawa S-I (2005) J Chem Phys 123:244507
Gamieldien MR, Strümpfer J, Naidoo KJ (2012) J Phys Chem B 116:324–331
Allesch M, Lightstone FC, Schwegler E, Galli G (2008) J Chem Phys 128:014501
Allesch M, Schwegler E, Galli G (2007) J Phys Chem B 111:1081–1089
Urahata S, Canuto S (1999) Chem Phys Lett 313:235–240
Tubergen MJ, Andrews AM, Kuczkowski RL (1993) J Phys Chem 97:7451–7457
Matsumoto Y, Honma K (2009) J Chem Phys 130:054311
Li Y, Liu X-H, Wang X-Y, Lou N-Q (1999) J Phys Chem A 103:2572–2579
Kumar A, Kołaski M, Kim KS (2008) J Chem Phys 128:034304
Sobolewski AL, Domcke W (2000) Chem Phys Lett 321:479–484
Frank I, Damianos K (2008) Chem Phys 343:347–352
Riley KE, Pitoňák M, Jurečka P, Hobza P (2010) Chem Rev 110:5023–5063
Hohenstein EG, Sherrill CD (2009) J Phys Chem A 113:878–886
Grimme S, Antony J, Ehrlich S, Krieg H (2010) J Chem Phys 132:154104
Goerigk L, Grimme S (2011) Phys Chem Chem Phys 13:6670–6688
Grimme S (2011) WIREs Comput Mol Sci 1:211–228
Burns LA, Vázquez-Mayagoitia Á, Sumpter BG, Sherrill CD (2011) J Chem Phys 134:084107
Chai J-D, Head-Gordon M (2008) Phys Chem Chem Phys 10:6615–6620
Chai J-D, Head-Gordon M (2008) J Chem Phys 128:084106
Tian B, Eriksson ESE, Eriksson LA (2010) J Chem Theory Comput 6:2086–2094
Kang YK, Byun BJ (2010) J Comput Chem 31:2915–2923
Simon S, Duran M (1996) J Chem Phys 105:11024–11031
Boys SF, Bernardi F (1970) Mol Phys 19:553–566
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR et al. (2010) Gaussian 09 C01. Gaussian Inc, Wallingford, CT. <http://www.gaussian.com>
Schwabe T, Grimme S (2007) Phys Chem Chem Phys 9:3397–3406
Zhao Y, Truhlar DG (2008) Acc Chem Res 41:157–167
Bader RFW (1991) Chem Rev 91:893–928
Bader RFW (1990) Atoms in molecules: a quantum theory. Clarendon, Oxford
Grabowski SJ, Ugalde JM (2010) J Phys Chem A 114:7223–7229
Keith TA, AIMAll, Version 10.03.25, <http://aim.tkgristmill.com>
Lu T, Chen F (2012) J Comput Chem 33:580–592
Lu T. Multiwfn, Version 2.3.3, <http://multiwfn.codeplex.com>
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899–926
Morokuma K (1971) J Chem Phys 55:1236–1244
Ziegler T, Rauk A (1977) Theor Chim Acta 46:1–10
Mitoraj MP, Michalak A, Ziegler T (2009) J Chem Theory Comput 5:962–975
Grimme S, Antony J, Schwabe T, Mück-Lichtenfeld C (2007) Org Biomol Chem 5:741–758
Baerends EJ, Autschbach J, Bashford D, Bérces A, Bickelhaupt FM, Bo C et al (2012) ADF version 2012.01, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands.<http://www.scm.com>
te Velde G, Bickelhaupt FM, Baerends EJ, Fonseca Guerra C, van Gisbergen SJA, Snijders JG, Ziegler T (2001) J Comput Chem 22:931–967
Bickelhaupt FM, Baerends EJ (2000) Kohn-Sham density functional theory: predicting and understanding chemistry. In: Lipkowitz KB, Boyd DB (eds) vol. 15 Rev Comput Chem. Wiley-VCH, New York, pp 1–86 doi:10.1002/9780470125922.ch1
Liu Z (2009) J Phys Chem A 113:6410–6414
Allen MP, Tildesley DJ (1987) Computer simulation of liquids. Clarendon, Oxford
Jorgensen WL, Maxwell DS, Tirado-Rives J (1996) J Am Chem Soc 117:11225–11236
Ponder JW. Tinker, version 4.2, <http://dasher.wustl.edu/tinker>
Mark P, Nilsson L (2001) J Phys Chem A 105:9954–9960
Berweger CD, van Gunsteren WF, Müller-Plathe F (1995) Chem Phys Lett 232:429–436
McDonald NA, Jorgensen WL (1998) J Phys Chem B 102:8049–8059
Cornell WD, Cieplak P, Bayly CI, Gould IR, Merz KM, Ferguson DM, Spellmeyer DC, Fox T, Caldwell JW, Kollman PA (1995) J Am Chem Soc 117:5179–5197
Joseph J, Jemmis ED (2007) J Am Chem Soc 129:4620–4632
Pluhácková K, Hobza P (2007) ChemPhysChem 8:1352–1356
Koch U, Popelier PLA (1995) J Phys Chem 99:9747–9754
Lipkowski P, Grabowski SJ, Robinson TL, Leszczynski J (2004) J Phys Chem A 108:10865–10872
Johnson ER, Keinan S, Mori-Sánchez P, Contreras-García J, Cohen AJ, Yang W (2010) J Am Chem Soc 132:6498–6506
Chocholoušová J, Špirko V, Hobza P (2004) Phys Chem Chem Phys 6:37–41
Alabugin IV, Manoharan M, Peabody S, Weinhold F (2003) J Am Chem Soc 125:5973–5987
Zhou P-P, Qiu W-Y (2009) J Phys Chem A 113:10306–10320
Rozas I (2007) Phys Chem Chem Phys 9:2782–2790
Raschke TM, Levitt M (2005) Proc Natl Acad Sci U S A 102:6777–6782
Chandler D (2005) Nature 437:640–647
Lide DR (ed) (2010) CRC handbook of chemistry and physics, 90th edn., (internet version 2010). CRC Press/Taylor and Francis, Boca Raton
Acknowledgments
Financial support by the National Natural Science Foundation of China (No. 21173069) as well as the Science and Technology Foundation of Guangdong Province, China (2010B060900084) are acknowledged. Authors also thank Dr. Tian Lu at the Institute of Chemical and Biological Technology, University of Science and Technology Beijing for helpful discussion. The computation of the Gaussian is supported by the School of Chemical and Environmental Sciences, Henan Normal University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, W., Jiao, J., Feng, H. et al. Natures of benzene-water and pyrrole-water interactions in the forms of σ and π types: theoretical studies from clusters to liquid mixture. J Mol Model 19, 1273–1283 (2013). https://doi.org/10.1007/s00894-012-1659-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1659-x