Abstract
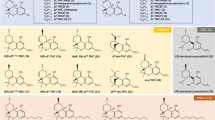
Cannabinoid receptor-1 (CB1) is widely expressed in the central nervous system and plays a vital role in regulating food intake and energy expenditure. CB1 antagonists such as Rimonabant have been used in clinic to inhibit food intake, and therefore reduce body weight in obese animals and humans. To investigate the binding modes of CB1 antagonists to the receptor, both receptor- and ligand-based methods were implemented in this study. At first, a pharmacophore model was generated based on 31 diverse CB1 antagonists collected from literature. A test set validation and a simulated virtual screening evaluation were then performed to verify the reliability and discriminating ability of the pharmacophore. Meanwhile, the homology model of CB1 receptor was constructed based on the crystal structure of human β 2 adrenergic receptor (β 2-AR). Several classical antagonists were then docked into the optimized homology model with induced fit docking method. A hydrogen bond between the antagonists and Lys192 on the third transmembrane helix of the receptor was formed in the docking study, which has proven to be critical for receptor-ligand interaction by biological experiments. The structure obtained from induced fit docking was then confirmed to be a reliable model for molecular docking from the result of the simulated virtual screening. The consistency between the pharmacophore and the homology structure further proved the previous observation. The built receptor structure and antagonists’ pharmacophore should be useful for the understanding of inhibitory mechanism and development of novel CB1 antagonists.






Similar content being viewed by others
References
Hanus L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, Kustanovich I, Mechoulam R (2001) 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci U S A 98(7):3662–3665. doi:10.1073/pnas.061029898
Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG (2002) International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 54(2):161–202
Boyd ST (2006) The endocannabinoid system. Pharmacotherapy 26(12 Pt 2):218S–221S. doi:10.1592/phco.26.12part2.218S
Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346(6284):561–564. doi:10.1038/346561a0
Lu ZL, Saldanha JW, Hulme EC (2002) Seven-transmembrane receptors: crystals clarify. Trends Pharmacol Sci 23(3):140–146
James PT, Leach R, Kalamara E, Shayeghi M (2001) The worldwide obesity epidemic. Obes Res 9:228s–233s
Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, Schaff HV (1997) Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med 337(9):581–588
Woods SC (2007) Role of the endocannabinoid system in regulating cardiovascular and metabolic risk factors. Am J Med 120(3 Suppl 1):S19–S25. doi:10.1016/j.amjmed.2007.01.004
Carai MA, Colombo G, Gessa GL (2005) Rimonabant: the first therapeutically relevant cannabinoid antagonist. Life Sci 77(19):2339–2350. doi:10.1016/j.lfs.2005.04.017
Wang HW, Duffy RA, Boykow GC, Chackalamannil S, Madison VS (2008) Identification of novel cannabinoid CB1 receptor antagonists by using virtual screening with a pharmacophore model. J Med Chem 51(8):2439–2446. doi:10.1021/Jm701519h
Murineddu G, Ruiu S, Loriga G, Manca I, Lazzari P, Reali R, Pani L, Toma L, Pinna GA (2005) Tricyclic pyrazoles. 3. Synthesis, biological evaluation, and molecular modeling of analogues of the cannabinoid antagonist 8-chloro-1-(2',4'-dichlorophenyl)-N-piperidin-1-yl-1,4,5,6-tetrahydrobenzo [6, 7]cyclohepta[1,2-c]pyrazole-3-carboxamide. J Med Chem 48(23):7351–7362. doi:10.1021/jm050317f
Krishnamurthy M, Li W, Moore BM (2004) Synthesis, biological evaluation, and structural studies on N1 and C5 substituted cycloalkyl analogues of the pyrazole class of CB1 and CB2 ligands. Bioorg Med Chem 12(2):393–404. doi:10.1016/j.bmc.2003.10.045
Katoch-Rouse R, Pavlova OA, Caulder T, Hoffman AF, Mukhin AG, Horti AG (2003) Synthesis, structure-activity relationship, and evaluation of SR141716 analogues: Development of central cannabinoid receptor ligands with lower lipophilicity. J Med Chem 46(4):642–645. doi:10.1021/Jm020157x
Shu H, Izenwasser S, Wade D, Stevens ED, Trudell ML (2009) Synthesis and CB1 cannabinoid receptor affinity of 4-alkoxycarbonyl-1,5-diaryl-1,2,3-triazoles. Bioorg Med Chem Lett 19(3):891–893. doi:10.1016/j.bmcl.2008.11.110
Muccioli GG, Martin D, Scriba GKE, Poppitz W, Poupaert JH, Wouters J, Lambert DM (2005) Substituted 5,5 '-diphenyl-2-thioxoimidazolidin-4-one as CB1 cannabinoid receptor ligands: Synthesis and pharmacological evaluation. J Med Chem 48(7):2509–2517. doi:10.1021/Jm049263k
Lan RX, Liu Q, Fan PS, Lin SY, Fernando SR, McCallion D, Pertwee R, Makriyannis A (1999) Structure-activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J Med Chem 42(4):769–776
Carpino PA, Griffith DA, Sakya S, Dow RL, Black SC, Hadcock JR, Iredale PA, Scott DO, Fichtner MW, Rose CR, Day R, Dibrino J, Butler M, DeBartolo DB, Dutcher D, Gautreau D, Lizano JS, O'Connor RE, Sands MA, Kelly-Sullivan D, Ward KM (2006) New bicyclic cannabinoid receptor-1 (CB1-R) antagonists. Bioorg Med Chem Lett 16(3):731–736. doi:10.1016/j.bmcl.2005.10.019
Ellsworth BA, Wang Y, Zhu YH, Pendri A, Gerritz SW, Sun C, Carlson KE, Kang LY, Baska RA, Yang YF, Huang Q, Burford NT, Cullen MJ, Johnghar S, Behnia K, Pelleymounter MA, Washburn WN, Ewing WR (2007) Discovery of pyrazine carboxamide CB1 antagonists: The introduction of a hydroxyl group improves the pharmaceutical properties and in vivo efficacy of the series. Bioorg Med Chem Lett 17(14):3978–3982. doi:10.1016/j.bmcl.2007.04.087
Foloppe N, Benwell K, Brooks TD, Kennett G, Knight AR, Misra A, Monck NJT (2009) Discovery and functional evaluation of diverse novel human CB1 receptor ligands. Bioorg Med Chem Lett 19(15):4183–4190. doi:10.1016/j.bmcl.2009.05.114
Smith RA, Fathi Z, Brown SE, Choi S, Fan J, Jenkins S, Kluender HC, Konkar A, Lavoie R, Mays R, Natoli J, O'Connor SJ, Ortiz AA, Podlogar B, Taing C, Tomlinson S, Tritto T, Zhang Z (2007) Constrained analogs of CB-1 antagonists: 1,5,6,7-Tetrahydro-4H-pyrrolo[3,2-c]pyridine-4-one derivatives. Bioorg Med Chem Lett 17(3):673–678. doi:10.1016/j.bmcl.2006.10.095
Lange JH, Kruse CG (2008) Cannabinoid CB1 receptor antagonists in therapeutic and structural perspectives. Chem Rec 8(3):156–168. doi:10.1002/tcr.20147
Griffith DA, Hadcock JR, Black SC, Iredale PA, Carpino PA, DaSilva-Jardine P, Day R, DiBrino J, Dow RL, Landis MS, O'Connor RE, Scott DO (2009) Discovery of 1-[9-(4-chlorophenyl)-8-(2-chlorophenyl)-9H-purin-6-yl]-4-ethylaminopiperi dine-4-carboxylic acid amide hydrochloride (CP-945,598), a novel, potent, and selective cannabinoid type 1 receptor antagonist. J Med Chem 52(2):234–237. doi:10.1021/jm8012932 10.1021/jm8012932
Dow RL, Carpino PA, Hadcock JR, Black SC, Iredale PA, DaSilva-Jardine P, Schneider SR, Paight ES, Griffith DA, Scott DO, O'Connor RE, Nduaka CI (2009) Discovery of 2-(2-chlorophenyl)-3-(4-chlorophenyl)-7-(2,2-difluoropropyl)-6,7-dihydro-2 H-pyrazolo[3,4-f][1,4]oxazepin-8(5H)-one (PF-514273), a novel, bicyclic lactam-based cannabinoid-1 receptor antagonist for the treatment of obesity. J Med Chem 52(9):2652–2655. doi:10.1021/jm900255t
Hurst DP, Lynch DL, Barnett-Norris J, Hyatt SM, Seltzman HH, Zhong M, Song ZH, Nie JJ, Lewis D, Reggio PH (2002) N-(Piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (SR141716A) interaction with LYS 3.28(192) is crucial for its inverse agonism at the cannabinoid CB1 receptor. Mol Pharmacol 62(6):1274–1287
Hurst D, Umejiego U, Lynch D, Seltzman H, Hyatt S, Roche M, McAllister S, Fleischer D, Kapur A, Abood M, Shi SP, Jones J, Lewis D, Reggio P (2006) Biarylpyrazole inverse agonists at the cannabinoid CB1 receptor: Importance of the C-3 carboxamide oxygen/lysine3.28(192) interaction. J Med Chem 49(20):5969–5987. doi:10.1021/Jm060446b
Shire D, Calandra B, Delpech M, Dumont X, Kaghad M, Le Fur G, Caput D, Ferrara P (1996) Structural features of the central cannabinoid CB1 receptor involved in the binding of the specific CB1 antagonist SR 141716A. J Biol Chem 271(12):6941–6946
Trillou CR, Arnone M, Delgorge C, Gonalons N, Keane P, Maffrand JP, Soubrie P (2003) Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol 284(2):R345–R353. doi:10.1152/ajpregu.00545.2002
Hagmann WK (2008) The discovery of taranabant, a selective cannabinoid-1 receptor inverse agonist for the treatment of obesity. Archiv Der Pharmazie 341(7):405–411. doi:10.1002/ardp. 200700255
Shim JY, Welsh WJ, Cartier E, Edwards JL, Howlett AC (2002) Molecular interaction of the antagonist N-(piperidin-1-yl)-5-(4-chlorophenyl)-1- (2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide with the CB1 cannabinoid receptor. J Med Chem 45(7):1447–1459
Tong WD, Hong HX, Xie Q, Shi LM, Fang H, Perkins R (2005) Assessing QSAR Limitations - A Regulatory Perspective. Curr Comput Aid Drug 1(2):195–205
Carlsson J, Coleman RG, Setola V, Irwin JJ, Fan H, Schlessinger A, Sali A, Roth BL, Shoichet BK (2011) Ligand discovery from a dopamine D(3) receptor homology model and crystal structure. Nat Chem Biol 7(11):769–778. doi:10.1038/Nchembio.662
Sun XQ, Li YZ, Li WH, Xu ZJ, Tang Y (2011) Computational investigation of interactions between human H(2) receptor and its agonists. J Mol Graph Model 29(5):693–701. doi:10.1016/j.jmgm.2010.12.001
Cheng FX, Xu ZJ, Liu GX, Tang Y (2010) Insights into binding modes of adenosine A(2B) antagonists with ligand-based and receptor-based methods. European Journal of Medicinal Chemistry 45(8):3459–3471. doi:10.1016/j.ejmech.2010.04.039
Cheng JX, Liu GX, Zhang J, Xu ZJ, Tang Y (2011) Insights into subtype selectivity of opioid agonists by ligand-based and structure-based methods. J Mol Model 17(3):477–493. doi:10.1007/s00894-010-0745-1
Lu C, Jin F, Li C, Li W, Liu G, Tang Y (2011) Insights into binding modes of 5-HT2c receptor antagonists with ligand-based and receptor-based methods. J Mol Model 17(10):2513–2523. doi:10.1007/s00894-010-0936-9
Jin F, Lu C, Sun X, Li W, Liu G, Tang Y (2011) Insights into the binding modes of human beta-adrenergic receptor agonists with ligand-based and receptor-based methods. Mol Divers 15(4):817–831. doi:10.1007/s11030-011-9311-8
Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC (2007) High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science 318(5854):1258–1265. doi:10.1126/science.1150577
Shim JY, Welsh WJ, Howlett AC (2003) Homology model of the CB1 cannabinoid receptor: Sites critical for nonclassical cannabinoid agonist interaction. Biopolymers 71(2):169–189. doi:10.1002/Bip. 10424
Salo OM, Lahtela-Kakkonen M, Gynther J, Jarvinen T, Poso A (2004) Development of a 3D model for the human cannabinoid CB1 receptor. J Med Chem 47(12):3048–3057. doi:10.1021/jm031052c
Montero C, Campillo NE, Goya P, Paez JA (2005) Homology models of the cannabinoid CB1 and CB2 receptors. A docking analysis study. Eur J Med Chem 40(1):75–83. doi:10.1016/j.ejmech.2004.10.002
Lin LS, Ha S, Ball RG, Tsou NN, Castonguay LA, Doss GA, Fong TM, Shen CP, Xiao JC, Goulet MT, Hagmann WK (2008) Conformational analysis and receptor docking of N-[(1S,2S)-3-(4-Chlorophenyl)-2-(3-cyanophenyl)-1-methylpropyl]-2-methyl-2-{[5-(trifluoromethyl)pyridin-2-yl]oxy}propanamide (taranabant, MK-0364), a novel, acyclic cannabinoid-1 receptor inverse agonist. J Med Chem 51(7):2108–2114. doi:10.1021/Jm7014974
Okada T, Sugihara M, Bondar AN, Elstner M, Entel P, Buss V (2004) The retinal conformation and its environment in rhodopsin in light of a new 2.2 A crystal structure. J Mol Biol 342(2):571–583. doi:10.1016/j.jmb.2004.07.044
Shim JY (2009) Transmembrane Helical Domain of the Cannabinoid CB1 Receptor. Biophys J 96(8):3251–3262. doi:10.1016/j.bpj.2008.12.3934
Zhang Y, Burgess JP, Brackeen M, Gilliam A, Mascarella SW, Page K, Seltzman HH, Thomas BF (2008) Conformationally constrained analogues of N-(piperidinyl)-5-(4-chlorophenyl)-1-(2,4- dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (SR141716): design, synthesis, computational analysis, and biological evaluations. J Med Chem 51(12):3526–3539. doi:10.1021/jm8000778
Catalyst User Guide, version 4.10 (Software Package), Accelrys, Inc, San Diego, CA, USA, 2005. http://accelrys.com/
Accelrys Software Inc., Discovery Studio Modeling Environment, Release 2.1,Accelrys, Inc, San Diego, CA, USA, 2004, http://accelrys.com/
Fang J, Shen J, Cheng FX, Xu ZJ, Liu GX, Tang Y (2011) Computational Insights into Ligand Selectivity of Estrogen Receptors from Pharmacophore Modeling. Mol Inform 30(6–7):539–549. doi:10.1002/minf.201000170
Boeckmann B, Bairoch A, Apweiler R, Blatter MC, Estreicher A, Gasteiger E, Martin MJ, Michoud K, O'Donovan C, Phan I, Pilbout S, Schneider M (2003) The SWISS-PROT protein knowledgebase and its supplement TrEMBL in 2003. Nucleic Acids Res 31(1):365–370
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000) The Protein Data Bank. Nucleic Acids Res 28(1):235–242
Schrödinger LLC (2008) Maestro, version 8.5, Schrödinger, LLC, New York, NY
Gross M (2011) Anarchy in the proteome. Chemistry World, Aug 2011, www.chemistryworld.org
Schrödinger LLC (2008) MacroModel, version 9.6, Schrödinger, LLC, New York, NY
Morris AL, MacArthur MW, Hutchinson EG, Thornton JM (1992) Stereochemical quality of protein structure coordinates. Proteins 12(4):345–364. doi:10.1002/prot.340120407
Schrödinger LLC (2005) LigPrep version 2.2, Schrödinger, LLC, New York, NY
Schrödinger LLC (2008) Glide version 5.0, Schrödinger, LLC, New York, NY
Wears RL, Lewis RJ (1999) Statistical models and Occam's razor. Acad Emerg Med 6(2):93–94
Kirchmair J, Markt P, Distinto S, Wolber G, Langer T (2008) Evaluation of the performance of 3D virtual screening protocols: RMSD comparisons, enrichment assessments, and decoy selection - What can we learn from earlier mistakes? J Comput Aided Mol Des 22:213–228
Jain AN, Nicholls A (2008) Recommendations for evaluation of computational methods. J ComputAided Mol Des 22:133–139
Telvekar VN, Patel KN (2011) Pharmacophore development and docking studies of the hiv-1 integrase inhibitors derived from N-methylpyrimidones, Dihydroxypyrimidines, and bicyclic pyrimidinones. Chem Biol Drug Des 78(1):150–160. doi:10.1111/j.1747-0285.2011.01130.x
Weber KC, de Lima EF, de Mello PH, da Silva ABF, Honorio KM (2010) Insights into the Molecular Requirements for the Anti-obesity Activity of a Series of CB1 Ligands. Chem Biol Drug Des 76(4):320–329. doi:10.1111/j.1747-0285.2010.01016.x
Baldwin JM, Schertler GF, Unger VM (1997) An alpha-carbon template for the transmembrane helices in the rhodopsin family of G-protein-coupled receptors. J Mol Biol 272(1):144–164. doi:10.1006/jmbi.1997.1240
McAllister SD, Rizvi G, Anavi-Goffer S, Hurst DP, Barnett-Norris J, Lynch DL, Reggio PH, Abood ME (2003) An aromatic microdomain at the cannabinoid CB1 receptor constitutes an agonist/inverse agonist binding region. J Med Chem 46(24):5139–5152. doi:10.1021/Jm0302647
Kim KH, Kim ND, Seong BL (2010) Pharmacophore-based virtual screening: a review of recent applications. Expert Opin Drug Dis 5(3):205–222. doi:10.1517/17460441003592072
Acknowledgments
This work was supported by the Program for New Century Excellent Talents in University (Grant NCET-08-0774), the 863 High-Tech Project (Grant 2006AA020404), the 111 Project (Grant B07023), and the Shanghai Committee of Science and Technology (Grant 11DZ2260600).
Author information
Authors and Affiliations
Corresponding author
Supplementary material available
The following information was provided in the Supporting Information: (1) Homology structure of CB1 receptor aligned with β 2-AR; (2) Ramachandran plot of the CB1 homology model; (3) Binding mode of SLV 319 with the homology model; (4) Structures and activities of test set compounds.
Rights and permissions
About this article
Cite this article
Kuang, G., Hu, G., Sun, X. et al. In silico investigation of interactions between human cannabinoid receptor-1 and its antagonists. J Mol Model 18, 3831–3845 (2012). https://doi.org/10.1007/s00894-012-1381-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1381-8