Abstract
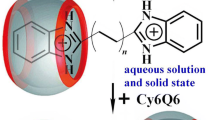
The binding of the laser dyes rhodamine B (RhB) and sulforhodamine B (kiton red S or KRS) to a cucurbit[7]uril (CB[7]) host has been investigated using density functional theory. Both guests (RhB and KRS) contain two N,N-diethylamino groups on a xanthene core. The lowest-energy structure of these host–guest complexes has one of the N,N-diethylamino groups encapsulated within the host cavity, that engenders C–H···O interactions with portals, while the remaining noninteracting diethylamino group resides outside the cavity. The 1H NMR chemical shifts derived using the gauge-independent atomic orbital method are consistent with those observed in experiments.

Binding modes of the fluorescent dyes RhB and KRS to a CB[7] host






Similar content being viewed by others
References
Behrend R, Meyer E, Rusche F (1905) I. Über Kondensationsprodukte aus Glycoluril und Formaldehyd. Liebigs Ann Chem 339:1–37. doi:10.1002/jlac.19053390102
Bhasikuttan AC, Mohanty J, Nau WM, Pal H (2007) Efficient fluorescence enhancement and cooperative binding of an organic dye in a supra-biomolecular host–protein assembly. Angew Chem Int Ed Engl 46:4120–4122. doi:10.1002/anie.200604757
Liu Y, LI CJ, Guo DS, Pan ZH, Li Z (2007) A comparative study of complexation of β-cyclodextrin, calix[4]arenesulfonate and cucurbit[7]uril with dye guests: fluorescence behavior and binding ability. Supramol Chem 19:517–523. doi:10.1080/10610270601145444
Marquez C, Hung F, Nau WM (2004) Cucurbiturils: molecular nanocapsules for time-resolved fluorescence-based assays. IEEE Trans Nanobiosci 3:39–45. doi:10.1109/TNB.2004.824269
Nau WM, Mohanty J (2005) Taming fluorescent dyes with cucurbituril. Int J Photoenergy 7:133–141. doi:10.1155/S1110662X05000206
Arunkumar E, Forbes CC, Smith BD (2005) Improving the properties of organic dyes by molecular encapsulation. Eur J Org Chem 19:4051–4059. doi:10.1002/ejoc.200500372
Halterman RL, Moore JL, Yakshe KA, Halterman JAI, Woodson KA (2010) Inclusion complexes of cationic xanthene dyes in cucurbit[7]uril. J Incl Phenom Macrocycl Chem 66:231–241. doi:10.1007/s10847-009-9615-9
Mohanty J, Jagtap K, Ray AK, Nau WM, Pal H (2010) Molecular encapsulation of fluorescent dyes affords efficient narrow-band dye laser operation in water. Chem Phys Chem 11:3333–3338. doi:10.1002/cphc.201000532
Pinjari RV, Khedkar JK, Gejji SP (2010) Cavity diameter and height of cyclodextrins and cucurbit[n]urils from the molecular electrostatic potential topography. J Incl Phenom Macrocycl Chem 66:371–380. doi:10.1007/s10847-009-9657-z
Pinjari RV, Gejji SP (2008) Electronic structure, molecular electrostatic potential, and NMR chemical shifts in cucurbit[n]urils (n = 5–8), ferrocene, and their complexes. J Phys Chem A 112:12679–12686. doi:10.1021/jp807268v
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE et al (2009) Gaussian 09. Gaussian Inc., Wallingford
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchanges. J Chem Phys 98:5648–5652. doi:10.1063/1.464913
Lee C, Yang W, Parr RG (1988) Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. doi:10.1103/PhysRevB.37.785
Wolinski K, Hilton JF, Pulay P (1990) Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J Am Chem Soc 112:8251–8260. doi:10.1021/ja00179a005
Mierts S, Scrocco E, Tomasi J (1981) Electrostatic interaction of a solute with a continuum. A direct utilization of ab initio molecular potentials for the prevision of solvent effects. J Chem Phys 55:117–129. doi:10.1016/0301-0104(81)85090-2
Kim J, Jung IS, Kim SY, Lee E, Kang JK, Sakamoto S, Yamaguchi K, Kim K (2000) New cucurbituril homologues: syntheses, isolation, characterization, and X-ray crystal structures of cucurbit[n]uril (n = 5, 7, and 8). J Am Chem Soc 122:540–541. doi:10.1021/ja993376p
Pinjari RV, Joshi KA, Gejji SP (2007) Theoretical studies on hydrogen bonding, NMR chemical shifts and electron density topography in α,β,γ-cyclodextrin conformers. J Phys Chem A 111:13583–13589. doi:10.1021/jp074539w
Acknowledgments
SPG acknowledges the University Grants Commission (UGC), New Delhi, India, for the research project F34-370/2008(SR). JKK thanks the UGC for the award of a meritorious student fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1720 kb)
Rights and permissions
About this article
Cite this article
Khedkar, J.K., Jagtap, K.K., Pinjari, R.V. et al. Binding of rhodamine B and kiton red S to cucurbit[7]uril: density functional investigations. J Mol Model 18, 3743–3750 (2012). https://doi.org/10.1007/s00894-012-1375-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1375-6