Abstract
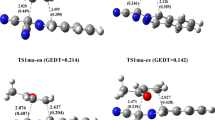
Density functional theory (DFT) was used to investigate the Mo-catalyzed intramolecular Pauson-Khand reaction of 3-allyloxy-1-propynylphosphonates. All intermediates and transition states were optimized completely at the B3LYP/6-31 G(d,p) level [LANL2DZ(f) for Mo]. In the Mo-catalyzed intramolecular Pauson-Khand reaction, the C–C oxidative cyclization reaction was the chirality-determining step, and the reductive elimination reaction was the rate-determining step. The carbonyl insertion reaction into the Mo–C(sp 3) bond was easier than into the Mo–C = C bond. And the dominant product predicted theoretically was of (S)-chirality, which agreed with experimental data. This reaction was solvent dependent, and toluene was the best among the three solvents toluene, CH3CN, and THF.

Density functional theory (DFT) study suggests that the Mo-catalyzed intramolecular Pauson-Khand reaction is solvent dependent, and that toluene is a better solvent than CH3CN or THF.











Similar content being viewed by others
References
Khand IU, Knox GR, Pauson PL, Watts WE, Foreman MI (1973) J Chem Soc Perkin Trans 1:975–977
Khand IU, Knox GR, Pauson PL, Watts WE, Foreman MI (1973) J Chem Soc Perkin Trans 1:977–981
Pauson PL, Khand IU, Ann NY (1977) Acad Sci 295:2–14
Pauson PL (1985) Tetrahedron 41:5855–5860
Murakami M, Amii H, Ito Y (1994) Nature 370:540–541
Blance-Urgoito J, Anorbe L, Pérez-Senano L, Domingcez G, Pérez-Castells J (2004) Chem Soc Rev 33:32–42
Brummond KM, Kent JL (2000) Tetrahedron 56:3263–3283
Chung YK (1999) Coord Chem Rev 188:297–341
Strübing D, Beller M (2006) Top Organomet Chem 18:165–178
Shibata T (2006) Adv Syn Catal 348:2328–2336
Shibata T, Toshida N, Takagi K (2002) Org Lett 4:1619–1621
Shibata T, Toshida N, Takagi K (2002) J Org Chem 68:7446–7450
Morimoto T, Fuji K, Tsutsumi K, Kakiuchi K (2002) J Am Chem Soc 124:3806–3807
Fuji K, Morimoto T, Tsutsumi K, Kakiuchi K (2003) Angew Chem Int Ed 42:2409–2411
Morimoto T, Kakiuchi K (2004) Angew Chem Int Ed 43:5580–5588
Kwong FY, Lee HW, Qiu L, Lam WH, Li YM, Kwong HL, Chan ASC (2005) Adv Syn Catal 347:1750–1754
Kent JL, Wan H, Brummond KM (1995) Tetrahedron Lett 36:2407–2410
Minami T, Okauchi T, Kouno R (2001) Synthesis 349–357
Dembitsky VM, Al Quntar AAA, Haj-Yehia A, Srebnik M (2005) MiniRev Org Chem 2:91–109
Maffei M (2004) Curr Org Synth 1:355–375
Quntar AA, Baum O, Reich R, Srebnik M (2004) Arch Pharm 337:76–80
Quntar AA, Gallily R, Katzavian G, Srebnik M (2007) Eur J Pharmacol 556:9–13
Cristau HJ, Gasc MB, Mbianda XY, Geze A (1996) Phosphorus Sulfur Silicon Relat Elem 111:159–159
Rivero MR, Carretero JC (2003) J Org Chem 68:2975–2978
Moradov D, Quntar AAAA, Youssef M, Smoum R, Rubinstein A, Srebrnik M (2009) J Org Chem 74:1029–1033
Imhof W, Anders E, Göbel A, Görls H (2003) Chem Eur J 9:1166–1181
Fjermestad T, Pericàs MA, Maseras F (2011) Chem Eur J 17:10050–10057
Wang C, Wu YD (2007) Organometallics 27:6152–6162
Baik MH, Mazumder S, Ricci P, Sawyer JR, Song YG, Wang H, Evans PA (2008) J Am Chem Soc 133:7621–7623
Baik MH, Mazumder S, Ricci P, Sawyer JR, Song YG, Wang H, Evans PA (2011) J Am Chem Soc 130:5821–5830
Gimbert Y, Lesage D, Milet A, Fournier F, Greene AE, Tabet JC (2003) Org Lett 5:4073–4075
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven JT, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PW, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, Revision B.05. Gaussian Inc, Pittsburgh PA
Parr RG, Yang W (1989) Density-functional theory of atoms and molecules. Oxford University Press, New York
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Hay PJ, Wadt WR (1985) J Chem Phys 82:270–283
Wadt WR, Hay PJ (1985) J Chem Phys 82:284–297
Hay PJ, Wadt WR (1985) J Chem Phys 82:299–310
Ehlers AW, Böhme M, Dapprich S, Gobbi A, Höllwarth A, Jonas V, Köhler KF, Stegmann R, Veldkamp A, Frenking G (1993) Chem Phys Lett 208:111–114
Gonzalez C, Schlegel HB (1990) J Phys Chem 94:5523–5527
Flükiger P, Lüthi HP, Portmann S, Weber J (2000–2002) MOLEKEL 4.3 Swiss Center for Scientific Computing, Manno. Switzerland
Portmann S, Lüthi HP (2000) Chimia 54:766–770
Carpenter JE, Weinhold F (1988) J Mol Struct THEOCHEM 169:41–50
Foster JP, Weinhold F (1980) J Am Chem Soc 102:7211–7218
Reed AE, Weinstock RB, Weinhold F (1985) J Chem Phys 83:735–746
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899–926
Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Weinhold F (2001) NBO 5.0, Theoretical Chemistry Institute, University of Wisconsin, Madison, WI
Marten B, Kim K, Cortis C, Friesner RA, Murphy RB, Ringnalda MN, Sitkoff D, Honig B (1996) J Phys Chem 100:11775–11788
Friesner RA, Murphy RB, Beachy MD, Ringnalda MN, Pollard WT, Dunietz BD, Cao YX (1999) J Phys Chem A 103:1913–1928
Miertus S, Tomasi J (1982) Chem Phys 65:239–245
Gorelsky SI, Lever ABP (2001) J Organomet Chem 635:187–196
Gorelsky SI (1997) AOMix: program for molecular orbital analysis. York University, Toronto, Canada; http://www.sf-chem.net/
Gorelsky SI, Ghosh S, Solomon EI (2006) J Am Chem Soc 128:278–290
Bader RFW (1990) Atoms in molecules, a quantum theory; International series of monographs in chemistry, vol 22. Oxford University Press, Oxford, UK
Biegler-König F, Schönbohm J, Derdau R, Bayles D, Bader RFW (2002) AIM 2000. Version 2.0, McMaster University
Acknowledgments
This work was supported by the Key Project of Science and Technology of the Ministry of Education, P. R. (grant No. 104263), Natural Science Foundation of Chongqing City, P. R. (grant No. CSTC-2004BA4024).
Supporting information
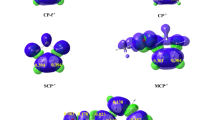
All energies, Wiberg bond orders P ij and electron density ρ (e·Å−3) at BCPs of some selected bonds are given by the supporting information.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Meng, Q., Li, M. Theoretical studies on the Mo-catalyzed asymmetric intramolecular Pauson-Khand-type [2 + 2 + 1] cycloadditions of 3-allyloxy-1-propynylphosphonates. J Mol Model 18, 3489–3499 (2012). https://doi.org/10.1007/s00894-012-1361-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1361-z