Abstract
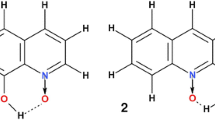
The 2-[1H]-pyridone/2-hydroxypyridine tautomeric pair and its 6-substituted complexes have been studied with the use of DFT(M05) method. The intermolecular interaction energy has been calculated and discussed in the light of secondary interaction concept. The attractive secondary interactions of O/NH and O/OH type and OH/NH and OH/OH repulsions have been analyzed in terms of stabilizing or destabilizing influence on intermolecular behavior. The transition states of the double proton transfer reaction have been found and the energy of activation has been determined. The activation energy of the proton transfer reaction, geometry of the complexes and transition states show NH2 and/or OH groups influence the properties of complexes and transition states. The HOMA index of aromaticity was applied to describe the π-electron delocalization in the heterocyclic rings.







Similar content being viewed by others
References
Prins LJ, Reinhoudt DN, Timmerman P (2001) Noncovalent synthesis using hydrogen bonding. Angew Chem Int Ed 40:2382–2426
Jeffrey GA (1997) An introduction to hydrogen bonding. Oxford University Press, Oxford
Brunsveld L, Folmer BJB, Meijer EW, Sijbesma RP (2001) Supramolecular polymers. Chem Rev 101:4071–4098. doi:10.1021/cr990125q
De Greef TFA, Smulders MMJ, Wolffs M, Schenning APHJ, Sijbesma RP, Meijer EW (2009) Supramolecular polymerization. Chem Rev 109:5687–5754. doi:10.1021/cr900181u
Schmidt J, Schmidt R, Wurthner F (2008) Synthesis, optical properties, and lfer analysis of solvent-dependent binding constants of hamilton-receptor-connected merocyanine chromophores. J Org Chem 73:6355–6362. doi:10.1021/jo801083b
Jiang YL, Gao X, Zhou G, Patel A, Javer A (2010) Selective recognition of uracil and its derivatives using a DNA repair enzyme structural mimic. J Org Chem 75:324–333. doi:10.1021/jo901862x
Kang SO, Day VW, Bowman-James K (2009) Fluoride: Solution- and solid-state structural binding probe. J Org Chem 75:277–283. doi:10.1021/jo901581w
Foces-Foces C, Echevarria A, Jagerovic N, Alkorta I, Elguero J, Langer U, Klein O, Minguet-Bonvehi M, Limbach H-H (2001) A solid-state nmr, x-ray diffraction, and ab initio computational study of hydrogen-bond structure and dynamics of pyrazole-4-carboxylic acid chains. J Am Chem Soc 123:7898–7906. doi:10.1021/ja002688l
Jonsson S, Arribas CS, Wendt OF, Siegel JS, Warnmark K (2005) Synthesis of 2-pyridone-fused 2, 2-bipyridine derivatives. An unexpectedly complex solid state structure of 3, 6-dimethyl-9 h–4, 5, 9-triazaphenanthren-10-one. Org Biomol Chem 3:996–1001
Zimmerman SC, Duerr BF (1992) Controlled molecular aggregation. 1. Cyclic trimerization via hydrogen bonding. J Org Chem 57:2215–2217
Beak P, Zeigler JM (1981) Molecular organization by hydrogen bonding: Juxtaposition of remote double bonds for photocyclization in a 2-pyridone dimer. J Org Chem 46:619–624
Wash PL, Maverick E, Chiefari J, Lightner DA (1997) Acid-amide intermolecular hydrogen bonding. J Am Chem Soc 119:3802–3806
Gallant M, Phan VMT, Wuest JD (1991) Hydrogen-bonded dimers. Direct study of the interconversion of pyridone dimers and hydroxy pyridine monomers by low-temperature nuclear magnetic resonance spectroscopy. J Am Chem Soc 113:721–723
Richards FM, Kundrot CE (1988) Identification of structural motifs from protein coordinate data: Secondary structure and first-level supersecondary structure. Proteins Struct Funct Genet 3:71–84
Kabsch W, Sander C (1983) Dictionary of protein secondary structure: Pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 22:2577–2637
Frishman D, Argos P (1995) Knowledge-based protein secondary structure assignment. Proteins Struct Funct Genet 23:566–579
Jeffery GA, Saenger W (1994) Hydrogen bonding in proteins. In: Hydrogen bonding in biological structures. Springer, Berlin, pp 351–394
Watson JD, Crick FHC (1953) Molecular structure of nucleic acids: A structure for deoxyribose nucleic acid. Nature 171:737–738
Watson JD, Crick FHC (1953) Genetical implications of the structure of deoxyribonucleic acid. Nature 171:964–967
Sieburth SM, McGee KF Jr (1999) Solvent-dependent stereoselectivity of bis-2-pyridone [4 + 4] photocycloaddition is due to h-bonded dimers. Org Lett 1:1775–1777
Clarke ML, Fuentes JA (2007) Self-assembly of organocatalysts: Fine-tuning organocatalytic reactions13. Angew Chem Int Ed Eng 46:930–933
Jorgensen WL, Pranata J (1990) Importance of secondary interactions in triply hydrogen bonded complexes: Guanine-cytosine vs uracil-2, 6-diaminopyridine. J Am Chem Soc 112:2008–2010
Alvares-Rua C, Garcia-Granda S, Goswami S, Mukherjee R, Dey S, Claramunt RM, Santa Maria MD, Rozas I, Jagerovic N, Alkorta I, Elguero J (2004) Multiple hydrogen bonds and tautomerism in naphthyridine derivatives. New J Chem 28:700–707
Xu W, Li X-C, Tan H, Chen G-J (2006) Theoretical study on stabilities of multiple hydrogen bonded dimers. Phys Chem Chem Phys 8:4427–4433
Ośmiałowski B (2009) Systematic investigation of 2, 7-dihydroxy-1, 8-naphthyridine dimerization - secondary interactions and tautomeric preferences calculations. J Mol Struct THEOCHEM 908:92–101
Luening U, Kuhl C, Uphoff A (2002) Four hydrogen bonds - ddaa, dada, daad and adda hydrogen bond motifs. Eur J Org Chem 2002:4063–4070
Szyc Ł, Guo J, Yang M, Dreyer J, Tolstoy PM, Nibbering ETJ, Czarnik-Matusewicz B, Elsaesser T, Limbach H-H (2010) The hydrogen-bonded 2-pyridone dimer model system. 1. Combined nmr and ft-ir spectroscopy study. J Phys Chem A 114:7749-7760. doi:10.1021/jp103630w
Yang M, Szyc Ł, Dreyer J, Nibbering ETJ, Elsaesser T The hydrogen-bonded 2-pyridone dimer model system. 2. Femtosecond mid-infrared pump-probe study. J Phys Chem A. doi:10.1021/jp108096y
Müller A, Losada M, Leutwyler S (2004) Ab initio benchmark study of (2-pyridone)2, a strongly bound doubly hydrogen-bonded dimer. J Phys Chem A 108:157–165
Forlani L, Cristoni G, Boga C, Todesco PE, Vecchio ED, Selva S, Monari M (2002) Reinvestigation of the tautomerism of some substituted 2-hydroxypyridines ARKIVOC 11:198-215
Yang HW, Craven BM (1998) Charge density study of 2-pyridone. Acta Crystallogr B 54:912–920. doi:10.1107/S0108768198006545
Held A, Pratt DW (1990) The 2-pyridone dimer, a model cis peptide. Gas-phase structure from high-resolution laser spectroscopy. J Am Chem Soc 112:8629–8630
Abdulla HI, El-Bermani MF (2001) Infrared studies of tautomerism in 2-hydroxypyridine 2-thiopyridine and 2-aminopyridine. Spectrochim Acta A 57:2659–2671
Bensaude O, Chevrier M, Dubois JE (1978) Lactim-lactam tautomeric equilibriums of 2-hydroxypyridines. 1. Cation binding, dimerization, and interconversion mechanism in aprotic solvents. A spectroscopic and temperature-jump kinetic study. J Am Chem Soc 100:7055–7060. doi:10.1021/ja00490a046
Bensaude O, Dreyfus M, Dodin G, Dubois JE (1977) Intramolecular nondissociative proton transfer in aqueous solutions of tautomeric heterocycles: A temperature-jump kinetic study. J Am Chem Soc 99(13):4438–4446. doi:10.1021/ja00455a037
Bensaude O, Chevrier M, Dubois J-E (1978) Influence of hydration upon tautomeric equilibria. Tetrahedron Lett 19:2221–2224
Hammes GG, Park AC (1969) Kinetic and thermodynamic studies of hydrogen bonding. J Am Chem Soc 91:956–961. doi:10.1021/ja01032a028
Hammes GG, Spivey HO (1966) A kinetic study of the hydrogen-bond dimerization of 2-pyridone1. J Am Chem Soc 88:1621–1625. doi:10.1021/ja00960a006
Wang J, Boyd RJ (1996) Tautomeric equilibria of hydroxypyridines in different solvents: An ab initio study. J Phys Chem 100:16141–16146. doi:10.1021/jp961295z
Wei CY, Chou PT, Hung FT (1997) Conjugated dual hydrogen bonds mediating 2-pyridone/2-hydroxypyridine tautomerism. J Phys Chem B 101:9119–9126
Katritzky AR, Ghiviriga I (1995) An NMR study of the tantomerism of 2-acylaminopyridines. J Chem Soc Perkin Trans 2:1651–1653
Krygowski TM, Pawlak D, Anulewicz R, Rasała D, Gawinecki R, Häfelinger G, Homsi MN, Kuske FKH (1996) Resonance interactions in the n-nitramino group. X-ray study of alpha-, beta- and gamma-nitraminopyridines. Acta Chem Scand 50:808–815
Gawinecki R, Raczyńska ED, Rasała D, Styrcz S (1997) Tautomeric and conformational preferences in nitraminopyridines: Comparison of theoretical and experimental data. Tetrahedron 53:17211–17220
Wu R, Brutschy B (2004) Infrared depletion spectroscopy and structure of the 2-aminopyridine dimer. J Phys Chem A 108:9715–9720
Müller A, Frey JA, Leutwyler S (2005) Probing the watson-crick, wobble, and sugar-edge hydrogen bond sites of uracil and thymine. J Phys Chem A 109:5055–5063
Müller A, Leutwyler S (2004) Nucleobase pair analogues 2-pyridone‚uracil, 2-pyridone‚thymine, and 2-pyridone‚5-fluorouracil: Hydrogen-bond strengths and intermolecular vibrations. J Phys Chem A 108:6156–6164
Rosciolo JR, Pratt DW (2003) Base pair analogs in the gas phase. Proc Natl Acad Sci USA 100:13752–13754
Hazra MK, Samanta AK, Chakraborty T (2007) Laser induced fluorescence spectroscopy of a mixed dimer between 2-pyridone and 7-azaindole. J Phys Chem A 111:7813–7818. doi:10.1021/jp072476b
Møller C, Plesset MS (1934) Note on an approximation treatment for many-electron systems. Phys Rev 46:618–622
Rozas I (2007) On the nature of hydrogen bonds: An overview on computational studies and a word about patterns. Phys Chem Chem Phys 9:2782–2790
Zhao Y, Truhlar DG (2006) Assessment of model chemistries for noncovalent interactions. J Chem Theor Comput 2:1009–1018
Zhao Y, Truhlar DG (2008) Exploring the limit of accuracy of the global hybrid meta density functional for main-group thermochemistry, kinetics, and noncovalent interactions. J Chem Theor Comput 4:1849–1868. doi:10.1021/ct800246v
Zhao Y, Truhlar DG (2008) The m06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four m06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241
González L, Mó O, Yáńez M, Elguero J (1996) Cooperative effects in water trimers. The performance of density functional approaches. J Mol Struct THEOCHEM 371:1–10
González L, Mó O, Yáńez M (1997) High-level ab initio versus dft calculations on (h2o2)2 and h2o2-h2o complexes as prototypes of multiple hydrogen bond systems. J Comput Chem 18:1124–1135
Mo O, Yanez M, Elguero J (1992) Cooperative (nonpairwise) effects in water trimers: An ab initio molecular orbital study. J Chem Phys 97:6628–6638
Mo O, Yanez M, Elguero J (1997) Study of the methanol trimer potential energy surface. J Chem Phys 107:3592–3601
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) J Comput Chem 14:1347–1363
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery J A, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, Revision e.01. Gaussian Inc, Pittsburgh, PA
Hunter C, Anderson H (2009) What is cooperativity? Angew Chem Int Ed 48:7488–7499. doi:10.1002/anie.200902490
Kuszewski J, Krygowski TM (1972) Definition of aromaticity basing on the harmonic oscillator model. Tetrahedron Lett 13:3839–3842
Acknowledgments
Financial support from the Polish Ministry of Science and Higher Education (grant no. N N204 174138) is gratefully acknowledged. The authors are very much indebted to the CYFRONET in Cracow for providing computer time and programs. The author thanks Prof. Ryszard Gawinecki for discussion and suggestions during composing this paper.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Materials
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ośmiałowski, B., Dobosz, R. The influence of secondary interactions on complex stability and double proton transfer reaction in 2-[1H]-pyridone/2-hydroxypyridine dimers. J Mol Model 17, 2491–2500 (2011). https://doi.org/10.1007/s00894-010-0934-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-010-0934-y