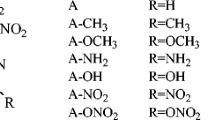Abstract
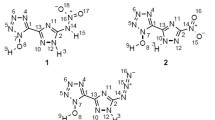
We have explored the geometric and electronic structures, band gap, thermodynamic properties, density, detonation velocity and detonation pressure of aminopolynitropyrazoles using the density functional theory (DFT) at the B3LYP/aug-cc-pVDZ level. The calculated detonation velocity and detonation pressure, stability and sensitivity of model compounds appear to be promising compared to the known explosives 3,4-dinitro-1 H-pyrazole (3,4-DNP), 3,5-dinitro-1 H-pyrazole (3,5-DNP), hexahydro-1,3,5-trinitro-1,3,5-triazinane (RDX) and octahydro-1,3,5,7-tetranitro-l,3,5,7-tetraazocane (HMX). The position of NH2 group in the polynitropyrazoles presumably determines the structure, stability, sensitivity, density, detonation velocity and detonation pressure.

Similar content being viewed by others
References
Kanishchev MI, Korneeva NV, Shevlev SA, Fainzil'berg AA (1988) Chem Heterocycl Compd 24:353–370
Shevelev SA, Dalinger IL, Shkineva TK, Ugrak BJ, Guleskaya MV, Kanishchev IJ (1993) Russ Chem Bull 42:1063–1068
Zaitsev AA, Dalinger IL, Shevelev SA (2009) Russ Chem Rev 78:589–627
Huttel R, Buchele F (1955) Chem Ber 88:1586–1590
Janssen JWAM, Habraken CL (1971) J Org Chem 36:3081–3083
Janssen JWAM, Koeners HJ, Kruse CG, Habraken CL (1973) J Org Chem 38:1777–1782
Janssen JWAM, Habraken CL, Louw R (1976) J Org Chem 41:1758–1762
Habraken CL, Poels EK (1977) J Org Chem 42:2893–2895
Habraken CL, Fernandes PC, Balaian S, van Erk KC (1970) Tetrahedraon Lett 479-480
Grimmett MR, Lim KHR (1978) Aust J Chem 31:689–691
Katritzky AR, Scriven EFV, Majundar S, Akhmedova RG, Akhmedov NG, Vakulenko AV (2005) ARKIVOC 3:179–191
Schmidt RD, Lee GS, Pagoria PF, Mitchell AR, Gilardi R (2001) J Heterocycl Chem 38:1227–1230
Dalinger IL, Vatsadze IA, Shkineva TK, Papova GP, Sheveleev SA (2010) Mendeleev Commun 20:253–254
Herve G, Roussel C, Graindorg H (2010) Angew Chem Int Edn 49:3177–3181
Li YF, Fan XW, Wang ZY, Ju XH (2009) J Mol Struct THEOCHEM 896:96–102
Ravi P, Gore GM, Venkatesan V, Tewari SP, Sikder AK (2010) J Hazard Mater 183:859–865
Depluech A, Cherville J (1978) Propellants Explos Pyrotech 3:169–175
Depluech A, Cherville J (1979) Propellants Explos Pyrotech 4:121–129
Xiao HM (1994) Molecular orbital theory of nitro compounds. Publishing House of Defense Industry, Peking
Kamlet MJ, Adolph HG (1979) Propellants Explos Pyrotech 4:30–34
Mullay J (1987) Propellants Explos Pyrotech 12:60–63
Politzer P, Murray JS (1995) Mol Phys 86:251–255
Murray JS, Concha MC, Politzer P (2009) Mol Phys 107:89–97
Rice BM, Hare JJ (2002) J Phys Chem A 106:1770–1783
Brinck T, Murray JS, Politzer P (1992) Mol Phys 76:609–617
Murray JS, Lane P, Politzer P (1995) Mol Phys 85:1–8
Murray JS, Lane P, Politzer P (1998) Mol Phys 93:187–194
Pospìŝil M, Vávra P, Concha MC, Murray JS, Politzer P (2010) J Mol Model 16:895–901
Zhang C, Shu Y, Huang Y, Zhao X, Dong H (2005) J Phys Chem B 109:8978–8982
Zhang C, Shu Y, Huang Y, Wang X (2005) J Energetic Mater 23:107–119
Zeman S (1999) J Energetic Mater 17:305–330
Zeman S (2006) J Hazard Mater 132:155–164
Zhi C, Cheng X (2010) Propellants Explos Pyrotech. doi:10.1002/prep.20090092
Zhang C (2009) J Hazard Mater 161:21–28
Zhang C, Shu Y, Wang X, Zhao X, Tan B, Peng R (2005) J Phys Chem A 109:6592–6596
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery AJ, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox J, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03, revision B.04. Gaussian Inc, Pittsburgh, PA
Njegic B, Gordon MS (2006) J Chem Phys 125:224102–224112
Hahre WJ, Radom L, PvR S, Pole JA (1986) Ab Initio Molecular Orbital Theory. Wiley, New York
Jursic BS (2000) J Mol Struct THEOCHEM 499:137–140
David RL (2003-2004) CRC Handbook of Chemistry and Physics, 84th edn. CRC Press
Materials Studio 4.1 (2004) Accelrys Inc. San Diego, CA
Wang GX, Shi CH, Gong XD, Zhu WH, Xiao HM (2009) J Hazard Mater 169:813–818
Kamlet MJ, Jacobs SJ (1968) J Chem Phys 48:23–35
Akhavan J (1998) Chemistry of explosives. The Royal Society of Chemistry, Cambridge
Stine JR (1981) Los Alamos national Laboratory’s Report, New Mexico
Ammon HL (2001) Struct Chem 12:205–212
Ehrlich HW (1960) Acta Crystallogr 13:946–952
Mondal T, Saritha B, Ghanta S, Roy TK, Mahapatra S, Prasad MD (2009) J Mol Struct THEOCHEM 897:42–47
Goh EM, Cho SG, Park BS (2000) J Def Tech Res 6:91–96
Politzer P, Martinez J, Murray JS, Concha MC (2009) Toro Labbe A 107:2095–2101
Fukui F, Yonezawa T, Shingu H (1952) J Chem Phys 20:722–725
Zhou Z, Parr RG (1990) J Am Chem Soc 112:5720–5724
Pearson RG (1989) J Org Chem 54:1423–1430
Skinner D, Olson D (1998) Propellants Explos Pyrotech 23:34–42
Wang G, Xiao H, Ju X, Gong X (2006) Propellants Explos Pyrotech 31:102–109
Wang G, Xiao H, Ju X, Gong X (2006) Propellants Explos Pyrotech 31:361–368
Acknowledgments
The first author acknowledges the sustaining financial support from Defence Research Development Organisation (DRDO), India through Advanced Centre of Research in High Energy Materials (ACRHEM). Department of Science and Technology (DST), India is thanked for the computational facility at the University of Hyderabad through Centre for Modeling Simulation and Design (CMSD).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ravi, P., Gore, G.M., Tewari, S.P. et al. Quantum chemical studies on the aminopolynitropyrazoles. J Mol Model 17, 2475–2484 (2011). https://doi.org/10.1007/s00894-010-0928-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-010-0928-9