Abstract
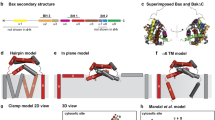
PB1-F2 is a recently described influenza A viral protein that induces apoptosis by binding with two mitochondrial membrane proteins, i.e. VDAC1 (outer membrane) and ANT3 (inner membrane). Knowledge of this binding mechanism could provide insights that would aid in the design of novel inhibitors against this protein. Therefore, to better understand these interactions, we have undertaken this study to model the PB1-F2 protein of the highly pathogenic influenza A virus subtype H5N1. Moreover, a model of human ANT3 was also established. The dynamics of the molecular interactions between the C-terminal region of PB1-F2 protein and VDAC1 and ANT3 were expounded by employing an in silico approach. Our results suggest the involvement of 12 amino acids of PB1-F2 protein, which form hydrophobic contacts with 22 amino acids of VDAC1. Of these, Leu64, Arg75 and Val76 were found to be crucial for mitochondrial targetting. In the case of the PB1-F2-ANT3 complex, 14 amino acids of ANT3 were found to make hydrophobic contacts with 9 amino acids of PB1-F2. Furthermore, two hydrogen bonds were predicted in both complexes PB1-F2/VDAC1 and PB1-F2/ANT3. This study reveals the molecular interactions required for PB1-F2-induced apoptosis and suggests a hypothetical model for future study.






Similar content being viewed by others
References
Khan AU (2006) Bioinformation 1(4):132–132
Chen W, Calvo PA, Malide D, Gibbs J, Schubert U, Bacik I, Basta SR, O’Neill, Schickli J, Palese P, Henklein P, Bennink JR, Yewdell JW(2001) Nat Med 7:1306–1312
Zamarin D, Ortigoza MB, Palese P (2006) J Virol 80:7976–7983
Zamarin D, Garcia-Sastre A, Xiao X, Wang R, Palese P (2005) P Los Pathog 1:e4
Gibbs JS, Malide D, Hornung F, Bennink JR, Yewdell JW (2003) J Virol 77:7214–7224
Yamada H, Chounan R, Higashi Y, Kurihara N, Kido H (2004) FEBS Lett 578:331–336
Thompson JD, Higgins DG, Gibson TJ (1994) Nucleic Acids Res 22:4673–4680
Sali A, Pottertone L, Yuan F, Vlijmen V, Karplus M (1995) Proteins 318-326
Brooks BR, Bruccoleri RE, Olafson BD, States DJ, Swaminathan S, Karplus M (1983) J Comp Chem 4:187–217
Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) J Appl Cryst 26:283–291
Wallace AC, Laskowski RA, Thornton JM (1995) Protein Eng 8:127–134
Ciminale V, Zotti L, D’Agostino DM, Ferro T, Casareto L, Franchini G, Bernardi P, Chieco-Bianchi L (1999) Oncogene 18:4505–4514
D’Agostino DM, Zotti L, Ferro T, Franchini G, Chieco-Bianchi L, Ciminale V (2000) AIDS Res Hum Retrovir 16:1765–1770
Ritchie DW, Kemp GJL (2000) Proteins 39:178–194
Acknowledgements
The authors acknowledge the facilities of the Distributed Information Sub-centre, Interdisciplinary Biotechnology Unit, AMU Aligarh. This work was supported by the DBT grant sanction no. BT/PR7507/BID/07/201/2006 to AUK. M.D. is the recipient of a DBT traineeship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Danishuddin, M., Khan, S.N. & Khan, A.U. Molecular interactions between mitochondrial membrane proteins and the C-terminal domain of PB1-F2: an in silico approach. J Mol Model 16, 535–541 (2010). https://doi.org/10.1007/s00894-009-0555-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-009-0555-5