Abstract
Ionization potential (IP), electron affinity (EA), dipole moment (μ) and electronic polarizability (α) of 1-, 3- and 6-nitrobenzo[a]pyrene isomers (1-NBaP, 3-NBaP, 6-NBaP) were determined by using density functional theory (DFT) and recent semiempirical PM6 methods. Calculated IP value remains almost constant along the series of isomers, while EA value depends on the nitro group position, increasing by ca. 0.2 eV on passing from 6- to 1-NBaP (or 3-NBaP) isomer. Stability, μ and α values decrease in the order 6-NBaP < 1-NBa ∼ 3-NBaP, the largest μ variation being predicted to be 1.5 D (30%) by DFT computations. The results obtained herein are consistent with the observed greater mutagenic activity of 3- and 1-NBaP in comparison to 6-NBaP isomer, suggesting that both binding to enzyme, which depends on electric properties, and reduction process, which is related to EA value may be crucial steps in the mutagenic mechanism of this series of isomers.

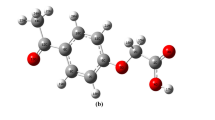
Structure and dipole moment vector of nitrobenzo[a]pyrene isomers



Similar content being viewed by others
References
Tokiwa H, Ohnishi Y (1986) Crit Rev Toxicol 17:23–60
Fu PP (1990) Drug Metab Rev 22:209–268
Chou MW, Heflich RH, Casciano DA, Miller DW, Freeman JP, Evans FE, Fu PP (1984) J Med Chem 27:1156–1161
Zhan D-J, Chiu L-H, Von Tungeln LS, Herreno-Saenz D, Cheng E, Evans FE, Heflich RH, Fu PP (1997) Mutat Res 379:43–52
Spain JC (1995) Ann Rev Microbiol 49:523–555
Parales RE, Ditty JL (2005) Curr Opin Biotechnol (2005) 16:315
Friemann R, Ivkovic-Jensen MM, Lessner DJ, Yu CL, Gibson DT, Parales RE, Eklund H (2005) J Mol Biol 348:1139–1151
Li YS, Fu PP, Church JS (2000) J Mol Struct 550–551:217–223
Pitts JN, Lokensgard DM, Harger W, Fisher TS, Mejia V, Schuler JJ, Scorziell GM, Katzenstein YA (1982) Mutat Res 103:241–249
Fukuhara K, Kurihara M, Miyata N (2001) J Am Chem Soc 123:8662–8666
Colvert KK, Fu PP (1986) Biochem Biophys Res Commun 141:245–250
Heflich RH, Unruh LE, Thornton-Manning JR, Von Tungeln LS, Fu PP (1989) Mutat Res 225:157–163
Hass BS, Heflich RH, Scho HM, Chou MW, Fu PP, Casciano DA (1986) Carcinogenesis 7:681–684
Horikawa K, Sera N, Murakami K, Sano N, Izumi K, Tokiwa H (1998) Toxicol Lett 98:51–58
Sera N, Kai M, Horikawa K, Fukuhara K, Miyata N, Tokiwa H (1991) Mutat Res 263:27–32
Jung H, Shaikh AU, Heflich RH, Fu PP (1991) Environ Mol Mutagen 17:169–180
Ishii S, Hisamatsu Y, Inazu K, Kobayashi T, Aika K-I (2000) Chemosphere 41:1809–1819
Warner SD, Lebuis A-M, Farant J-P, Butler IS (2003) J Chem Cryst 33:213–217
Onchoke KK, Hadad CM, Dutta PK (2006) J Phys Chem A 110:76–84
Dyker G, Kadzimirsz D, Thoene A (2003) Eur J Org Chem 16:3162–3166
Vance WA, Okamoto HS, Wang YY (1988) In: King CM, Romano LJ, Schuetzle D (eds) Carcinogenic and mutagenic responses to aromatic amines and nitroarenes. Elsevier, New York, pp 291–302
Debnath AK, Lopez de Compadre RL, Debnath G, Shusterman AJ, Hansch C (1991) J Med Chem 34:786–797
Becke AD (1993) J Chem Phys 98:1372–1377
Lee C, Yang AD, Parr RG (1988) Phys Rev B 37:785–789
Stewart JJP (2007) J Mol Model 13:1173–1213
Hehre WJ, Radom L, Schleyer PvR, Pople JA (1986) Ab initio molecular orbital theory. Wiley, New York
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899, and references therein
De Proft F, Martin JML, Geerlings P (1996) Chem Phys Lett 250:393–401
Stewart JJP, MOPAC 2007, Stewart Computational Chemistry, Colorado Springs, CO, USA, http://OpenMOPAC.net
Karna SP, Dupuis M (1991) J Comput Chem 12:487–504
Zerner MC (1991) In: Lipkowitz KB, Boyd DB (eds) Review computational chemistry, Vol. 2. VCH, New York, pp 313–366
Orr BJ, Ward JF (1971) Mol Phys 20:513–526
Modelli A, Mussoni L, Fabbri D (2006) J Phys Chem A 110:6482–6486
Modelli A, Jones D (2006) J Phys Chem A 110:13195–13201
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03, Revision B.03. Gaussian Inc, Pittsburgh PA
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL, Dupuis M, Montgomery JA Jr (1993) J Comput Chem 14:1347–1363
Koopmans T (1933) Physica 1:104–113
Klopman G, Tonucci DA, Holloway M Rosenkranz HS (1984) Mutat Res 126:139–144
Maynard AT, Pedersen LG, Posner HS, McKinney JD (1986) Mol Pharmacol 29:629–636
Lopez de Compadre RL, Shusterman AJ, Hansch C (1988) Int J Quantum Chem 34:91–101
Onchoke KK, Hadad CM, Dutta PK (2004) Polycyclic Aromat Compd 24:37–64
Takamura-Enya T, Suzuki H, Hisamatsu Y (2006) Mutagenesis 21:399–404
Heinis T, Chowdhury S, Kebarle P (1993) Org Mass Spectrom 28:358–365
Akiyama I, Li KC, LeBreton PR, Fu PP, Harvey RG (1979) J Phys Chem 83:2997–3003
Desfrancois C, Periquet V, Lyapustina SA, Lippa TP, Robinson DW, Bowen KH, Nonaka H (1999) J Chem Phys 111:4569–4576
Kimura K, Katsumata S, Achiba Y, Yamazaki T, Iwata S (1981) In: Handbook of HeI Photoelectron Spectra of Fundamental Organic Compounds. Ionization energies, Ab initio assignments, and valence electronic structure for 200 molecules. Japan Scientific Soc. Press, Tokyo
Crocker L, Wang TB, Kebarle P (1993) J Am Chem Soc 115:7818–7822
Klasinc L, Kovac B, Guesten H (1983) Pure Appl Chem 55:289–298
Ames BN, McCann J, Yamasaki E (1975) Mutat Res 31:347–364
Maron DM, Ames BN (1983) Mutat Res 113:173–215
Watanabe M, Ishidate Jr M, Nohmi T (1990) Mutat Res 234:337–348
Rosenkranz HS, Mermelstein R (1983) Mutat Res 114:217–267
Chattaraj PK, Sarkar U, Roy DR (2006) Chem Rev 106:2065–2091, and references therein
Pearson RG (1993) Acc Chem Res 26:250–255
Torrent-Sucarrat M, Luis JM, Duran M, Solà M (2001) J Am Chem Soc 123:7951–7952
Senthilkumar K, Kolandaivel P (2003) Comput Biol Chem 27:173–183
Selvarengan P, Kolandaivel P (2005) Bioorg Chem 33:253–263
Alparone A, Millefiori A, Millefiori S (2005) Chem Phys 312:261–274
Staikova M, Wania F, Donaldson DJ (2004) Atmos Environ 38:213–225, and references therein
Smyth CP (1955) Dielectric Behaviour and Structure. McGraw-Hill, New York
Velders GJM, Gillet JM, Becker PJ, Feil D (1991) J Phys Chem 95:8601–8608
McKinney JD (1989) Environ Health Perspect 82:323–336
Fraschini E, Bonati L, Pitea D (1996) J Phys Chem 100:10564–10569
Hirokawa S, Imasaka T, Imasaka T (2005) Chem Res Toxicol 18:232–238
Librando V, Alparone A (2007) Environ Sci Technol 41:1646–1652
Librando V, Alparone A (2007) Polycyclic Aromat Compd 27:65–94
Karelson M, Lobanov VS, Katritzky AR (1996) Chem Rev 96:1027–1044, and references therein
Singer KD, Garito AF (1981) J Chem Phys 75:3572–3580
Janssen RHC, Theodorou DN, Raptis S, Papadopoulos MG (1999) J Chem Phys 111:9711–9719
Chattaraj PK, Sengupta S (1996) J Phys Chem 100:16126–16130
Minisini B, Fayet G, Tsobnang F, Bardeau JF (2007) J Mol Model 13:1227–1235
Yu S, Herreno-Saenz D, Miller DW, Heflich RH, Kadlubar FF, Fu PP (1992) Mutat Res 283:45–52
Librando V, Alparone A (in press) J Hazard Mater , DOI 1016/j.jhazmat.20020
Acknowledgements
This work was carried out in the framework of the RIC action of the Project No. 1999/IT.16.1.PO.011/3.13/7.2.4/339 PROT. 238, “Formazione per la ricerca nel campo della bonifica dei siti contaminate” POR Sicilia 2000–2006, Asse: III Misura: 3.13.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Librando, V., Alparone, A. & Tomaselli, G. Electronic properties of some nitrobenzo[a]pyrene isomers: a possible relationship to mutagenic activity. J Mol Model 14, 489–497 (2008). https://doi.org/10.1007/s00894-008-0297-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-008-0297-9