Abstract
The potential of various organic species to catalyze epoxidation of ethene by hydrogen peroxide is explored with B3LYP/6-31G* DFT calculations.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrogen peroxide, 1, is a cheap and environmentally-friendly oxidant, since the only by-product is water, but it is not reactive enough for many applications. Catalysts to promote the epoxidation of alkenes, oxidation of sulfides, etc., by hydrogen peroxide are potentially of great value. While there are many examples of promotion of H2O2 reactions by transition metal ions, in this paper we discuss a different approach, based on the following simple idea. Water oxide [1], 2, is unknown, but would be expected to be an extremely powerful oxidant. Although water oxide is never likely to be involved as a reaction intermediate, a compound that could remove a proton from one oxygen of hydrogen peroxide as it attacked a substrate, while more or less simultaneously donating a proton to the other oxygen, thus creating a water molecule, could be a powerful catalyst for reactions of H2O2, as shown for a hypothetical RC(X)YH catalyst in 3 below.
Traditionally, alkene epoxidation has been carried out with peroxycarboxylic acids, although many other methods have been developed in recent years, especially for enantioselective epoxidation. Epoxidation with peroxycarboxylic acids is not very sensitive to solvent polarity and the accepted “butterfly” mechanism [2, 3] is concerted and involves a complex series of electron shifts [4, 5], as shown in 4 above. In this paper the process shown in 5 above for epoxidation of ethene is modeled, and the energetics are compared with those calculated for 4. The main objective is to define the best choice of proton transfer catalyst, but we also explore the potential for further hydrogen-bonding assistance, with a view to the eventual design of hosts that might catalyze hydrogen peroxide reactions with control of stereochemistry. Since the dielectric properties inside the host cannot be predicted, we have restricted our study to the gas phase, and have not attempted to model environmental effects.

Computational methods
All DFT calculations were performed with the Jaguar program package [6], using Becke’s three-parameter exchange functional [7] with the correlation functional of Lee, Yang and Parr (B3LYP) [8]. All species were characterized by full geometry optimization with the standard 6-31G* basis set, and minima and transition states were characterized by analytical frequency calculations. The single imaginary frequency of the transition states was animated in the Spartan ’04 program [9], and it was confirmed that this vibration involved the stretching of the O–O bond with one oxygen moving more or less symmetrically towards the alkene-carbon atoms, and with one hydrogen moving towards the putative water molecule. IRC calculations were carried out in Spartan ’04 for the reactions catalyzed by one and two formic acid molecules, and these confirmed that the transition states reported below do evolve to the starting complexes and epoxide products.
Protein Database (PDB) files for the species illustrated in the Figures are available as Electronic Supplementary Material.
Results and discussion
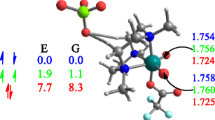
What form should the proton acceptor/proton donor catalyst take? Optimization of a complex of formic acid with hydrogen peroxide leads to the structure shown in Fig. 1a. The two hydrogen bonds are close to linear and of comparable length. Thus, the RC(X)YH species shown in 3 and 5 above appears to be a good starting point for catalysis design. Some alternative geometries for the catalyst are explored later.
Transition states have been located at the B3LYP/6-31G* level for the epoxidation of ethene by hydrogen peroxide catalyzed by various HC(X)YH species. Activation parameters calculated relative to ethene and an optimized complex of hydrogen peroxide with the catalyst are given in Table 1 and the transition state for the case of formic acid is shown in Fig. 1b. As can be seen, the latent epoxide ring is more or less perpendicular to the general plane containing the hydrogen peroxide and formic acid molecules; this type of transition-state geometry has been described as “spiro”, as opposed to “planar”.
A priori, it is not obvious whether it is desirable to have a strongly basic X-group to promote deprotonation of the epoxidizing oxygen atom or to go for a strongly acidic Y–H group to protonate the developing hydroxide ion. However, it can be seen from Table 1 that more acidic species are better catalysts, although methanesulfonic acid is not a significant improvement on trifluoroacetic acid. Proton donation to hydrogen peroxide is clearly more important than proton removal in the transition state. This is reasonable on the grounds that normal alkenes like ethene are attacked by electrophilic reagents. Full proton transfer would create H3O2 + [10–12], a powerfully electrophilic species that is formed in solutions of hydrogen peroxide in very strong acids that is known to be an extremely powerful (but very indiscriminate) oxidants [10–14].
Further evidence supporting the importance of proton transfer to the non-epoxidizing oxygen atom may be gleaned from examination of the geometry of the transition states. Thus, in the transition state for epoxidation catalyzed by formic acid, the OH bond in formic acid has lengthened to 1.13 Å, compared with 1.00 Å in the initial complex, and the distance from this proton to the oxygen atom in H2O2 has shortened from 1.77 to 1.32 Å. Furthermore, animation of the single imaginary frequency shows that motion of this proton is strongly coupled to the O–O stretching process. On the other hand, proton loss from the epoxidizing oxygen atom of the hydrogen peroxide has hardly begun at the transition state, since the OH bond has only stretched by 0.02 Å, although the distance from the proton to the carbonyl group of formic acid has shortened from 1.81 to 1.63 Å.
In order to provide some calibration of the activation parameters in Table 1, the transition state for epoxidation of ethene by peroxyformic acid was optimized using Jaguar. The accepted [15] ΔH‡ value was reproduced and ΔD;‡ was found to be 101.8 kJ mol−1 (see Table 1). This transition state has been the focus of much work [16, 17], with lively debate concerning spiro vs planar transition state geometries. As a result of high level ab initio and CASSCF calculations [15], it now appears that the spiro geometry is preferred, and that the economical B3LYP DFT method is a useful and reasonably accurate method that can be applied to studies of larger systems. The corresponding epoxidation of ethene with peroxytrifluoroacetic acid (see Table 1) resulted in lowered activation parameters, in qualitative agreement with the known high reactivity of CF3CO3H.
Comparison of the activation parameters for the mechanism shown in 5 (Table 1) with those for epoxidation via 4 with the corresponding peroxyacids, shows that the catalyzed process to be poorer by about 15 kJ mol−1 in ΔH‡. The catalyzed process 5 with carboxylic acids as catalysts is clearly a reasonable possibility, worthy of further optimization. When carboxylic acids are mixed with hydrogen peroxide, an equilibrium is set up involving formation of the peroxycarboxylic acid. Equilibration is quite slow, however, taking about 1 h at ambient temperature when 30% H2O2 is added to a large excess of 88% HCO2H [18, 19]. Since this reaction almost certainly involves nucleophilic attack at the carbonyl group of the carboxylic acid, equilibration is much slower for acetic and other simple carboxylic acids [20], although it could be faster for strong acids like CF3CO2H. The slow rates for this equilibration rule out its incorporation into a viable catalytic cycle. Most discussions of the promotion of hydrogen peroxide reactions by acids concentrate on the formation of new peroxidic species [21], rather than the proton transfer process proposed here, although there have been several recent reports of electrophilic activation of hydrogen peroxide by perfluorinated alcohols [22–24] and phenol [25]. In the absence of strong acids, it is likely that this activation involves clusters of strongly hydrogen-bonded solvent molecules, as suggested by Berkessel for catalysis by HFIP (1,1,1,3,3,3-hexafluoro-2-propanol) [24].
While the RC(X)YH species shown in 3 and 5 above appears to be a good starting point for catalysis design, it seemed desirable to see whether separating the proton-donating and proton-accepting groups by more (or fewer) atoms would offer any significant advantages. For a reliable comparison with carboxylic acids, the catalyst must be symmetrical with respect to the proton exchange. Thus, squaric acid derivatives, 6, are unsatisfactory, although they might be attractive in other respects. Derivatives of pyrazole 7 and of 2-hydroxycyclopentadienone 8 were selected for comparison with carboxylic acids. Tropolone 9 is of course much more stable and readily available than 8 but it forms a strong intramolecular H-bond, as do enolized β-diketones, and so these are unlikely to be good catalysts. In view of the clear importance of acidity for catalysis, we sought derivatives whose anions were of comparable proton affinity to formate and trifluoroacetate. The results are summarized in Table 1

.
While 8, R 1=H, R 2=CN yields the lowest value for ΔH‡ so far, this is due to its extreme acidity, rather than any superiority derived from having two carbons separating the proton donating and proton accepting groups. This conclusion is backed up by examination of the transition state with 8, R 1=H, R 2=CN as catalyst; the proton transfer from the catalyst is more or less complete, so this is almost a reaction of H3O2 +. Comparison based on relative PA-values shows that the carboxylic acids are somewhat superior to the 2-hydroxycyclopentadienone derivatives with the pyrazole derivatives a very poor third. Thus, the original catalyst design appears to be the best choice.
Extra hydrogen bonding: towards catalytic hosts for H2O2
The water molecule that is created in the catalyzed process should be stabilized by the provision of further hydrogen bonding, especially by proton donation to the oxygen. This is a likely component of the catalysis by perfluorinated alcohols and phenol cited above. Since our eventual aim is the design of hosts for H2O2 which might catalyze its reactions and ultimately provide steric control of the stereochemistry of epoxidation, we sought extra stabilization of the transition state associated with a well-defined geometry.
A second formic acid was therefore positioned to take up this role (the formic acid that carries out the proton transfer role will be called the primary acid). First, we optimized hydrogen peroxide complexed to two formic acid molecules This structure is shown in Fig. 2a. Ethene was then introduced, and a transition state located via QST-guided calculation in Jaguar, Fig. 2b. The activation parameters, ΔH‡ 67.7 and ΔG‡ 109.8 kJ mol−1 relative to ethene and the complex of hydrogen peroxide with two formic acid molecules, represent a significant improvement on the process catalyzed by a single formic acid, suggesting that hosts based on this design could form the basis of a successful process. In order to explore the optimum acidity properties of the two carboxylic acids, trifluoromethyl substituents were introduced in both positions. The results are summarized in Table 2.
(a) Hydrogen peroxide complexed to two formic acid molecules; (b) Transition state for epoxidation of ethane by hydrogen peroxide catalyzed by two formic acid molecules. The “primary” acid which takes part in proton transfer appears at the lower right while the “secondary” acid which contributes through static H-bonding appears at the lower left
As expected, two trifluoroacetic acids provide the best catalysis, but it is surprising that ΔH‡ is lower when a single trifluoroacetic acid is used in the secondary role, rather than the primary role. A possible explanation of this is that the primary acid molecule must act as both proton donor and acceptor, while that in the secondary role is a pure proton donor, since the hydrogen bond from the incipient water molecule to the carbonyl group of this acid is very long and weak.
Conclusions and implications for host design
In the H2O2·2HCO2H complex, the carbonyl carbons are separated by 6.30 Å, and the two H–C bonds subtend an angle of 160°. These parameters remain fairly constant as CF3 groups are introduced. In the transition state for epoxidation of ethene in the presence of two formic acids, the separation of the carbonyl carbons is reduced to 5.97 Å, and two H–C bonds subtend a smaller angle of 115°. As CF3 groups are introduced, this angle remains fairly constant, but the C…C distance decreases significantly, to 5.69 Å in the transition state involving two trifluoroacetic acids.
These geometrical parameters provide a useful guide to the design of potential hosts; the separation of around 6 Å requires quite a large host, but not so big as to be outside the scope of reliable calculations and further exploration is underway. Clearly it would be desirable to design a host which was somewhat strained as the H2O2 complex, but where this strain would be relieved as the transition state was attained.
References
Oiestad EL, Harvey JN, Uggerud E (2000) J Phys Chem A 104:8382–8388
Bartlett PD (1950) Record of Chemical Progress 11:47–51
Bartlett PD (1957) Record of Chemical Progress 18:111
Herges R (1994) J Chem Inf Comput Sci 34:91–102
Herges R (1994) Angew Chem-Int Edit Engl 33:255–276
Jaguar (2002) Jaguar 4.2 edn. Schrödinger Inc, Portland OR, USA
Becke ADJ (1993) Chem Phys 98:5648–5652
Lee CT, Yang WT, Parr RG (1988) Phys Rev B 37:785–789
Spartan ’04 ed (2004) Wavefunction Inc, Irvine CA, USA
Alder RW, Whiting MC (1964) J Chem Soc 4707–4712
Christe KO, Wilson WW, Curtis EC (1979) Inorg Chem 18:2578–2586
Olah GA, Berrier AL, Prakash GKS (1982) J Am Chem Soc 104:2373–2376
Olah GA, Yoneda N, Parker DG (1977) J Am Chem Soc 99:483–488
Olah GA, Parker DG, Yoneda N (1978) Angew Chem-Int Edit Engl 17:909–931
Bach RD, Glukhovtsev MN, Gonzalez C (1998) J Am Chem Soc 120:9902–9910
Houk KN, Liu J, DeMello NC, Condroski KR (1997) J Am Chem Soc 119:10147–10152
Freccero M, Gandolfi R, Sarzi-Amade M, Rastelli A (2004) J Org Chem 69:7479–7485
Toennies G, Homiller RP (1942) J Am Chem Soc 64:3054–3056
Monger JM, Redlich O (1956) J Phys Chem 60:797–799
Swern D (1949) Chem Rev 45:1–68
Vayssie S, Elias H (1997) Liebigs Ann-Recl 2567–2572
Neimann K, Neumann R (2000) Org Lett 2:2861–2863
Berkessel A, Andreae MRM (2001) Tetrahedron Lett 42:2293–2295
Berkessel A, Adrio JA (2004) Adv Synth Catal 346:275–280
Wahlen J, de Vos DE, Jacobs PA (2003) Org Lett 5:1777–1780
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Dr. Paul von Ragué Schleyer on the occasion of his 75th birthday
Electronic supplementary material
These files are unfortunately not in the Publisher's archive anymore:
-
(PDB 1 kb)
-
(PDB 2 kb)
-
(PDB 1 kb)
-
(PDB 2 kb)
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Alder, R.W., Davis, A.P. The design of organic catalysis for epoxidation by hydrogen peroxide. J Mol Model 12, 649–652 (2006). https://doi.org/10.1007/s00894-005-0044-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-005-0044-4