Abstract
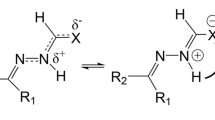
The geometrical structures of phenylthiosemicarbazone (HAPhTSC) conformers have been obtained by geometry optimizations using density functional theory (DFT) calculations at the B3LYP/6-31G(d) and B3LYP/6-311G(d,p) levels of theory. Six thioamino and 24 thioimino tautomers of HAPhTSC have been found. Six tautomerization reactions between thioamino and thioimino tautomers occurring via transition states and their corresponding activation energies have been obtained. Conformational pathways for tautomerizations and interconversions of HAPhTSC conformers have been presented. Tautomerization between the most stable species of thioamino (Atttcc) and its thioimino (Itttcct) tautomer is an endothermic reaction, ΔH0=18.17 kcal mol−1 and its log K=−13.74, at 298.15 K. Thermodynamic quantities of tautomerizations, interconversions of HAPhTSC conformers and their equilibrium constants are reported. The geometry of the zinc complex with HAPhTSC, found as a Zn(HAPhTSC)2Cl2 structure, has been obtained using B3LYP/6-31G(d) calculations. Binding of the Zn(HAPhTSC)2Cl2 complex is an exothermic and spontaneous reaction.
Figure Conformational notation defined as a name consisting of a letter “A” for a thioamino tautomer followed by “c” for cis or “t” for trans isomerism of five dihedral angles of χ(C4-C3-C2-N3), ϕ(C3-C2-N3-N2), ψ(C2-N3-N2-C1), θ(N3-N2-C1-N1) and ω(N2-C1-N1-H2), serially, or a letter “I” for b thioimino tautomer followed by “c” for cis or “t” for trans isomerism of six dihedral angles of χ(C4-C3-C2-N3), ϕ(C3-C2-N3-N2), ψ(C2-N3-N2-C1), θ(N3-N2-C1-N1), ω(N2-C1-N1-H2) and δ (N2-C1-S-H1), serially.









Similar content being viewed by others
References
Kolb VM, Stupar JW, Janota TE, Duax WL (1989) J Org Chem 54:2341–2346
Tian YP, Duan CY, Lu ZL, You XZ (1996) Polyhedron 15:2263–2271
Bresolin L, Burrow RA, Hörner M, Bermejo E, Castiñeiras A (1997) Polyhedron 16:3947–3951
Basuli F, Ruf M, Pierpont CG, Bhattacharya S (1998) Inorg Chem 37:6113–6116
Basuli F, Peng SM, Bhattacharya S (2000) Inorg Chem 39:1120–1127
Basuli F, Peng SM, Bhattacharya S (2001) Inorg Chem 40:1126–1133
Gupta P, Basuli F, Peng SM, Lee GH, Bhattacharya S (2003) Inorg Chem 42:2069–2074
Pal I, Dutta S, Basuli F, Goverdhan S, Peng SM, Lee GH, Bhattacharya S (2003) Inorg Chem 42:4338–4345
de Sousa GF, Deflon VM, Niquet E (2004) J Mol Struct 687:17–21
Kasuga NC, Sekino K, Koumo C, Shimada N, Ishikawa M, Nomiya K (2001) J Inorg Biochem 84:55–65
Babu RR, Vijayan N, Gopalakrishnan R, Rramasamy P (2002) J Crystal Growth 240:545–548
Ruangpornvisuti V, Pulpoka B, Tuntulani T, Thipyapong K, Suksai C (2002) Bull Korean Chem Soc 23:555–562
Thipyapong K, Arano Y, Ruangpornvisuti V (2004) J Mol Struct (Theochem) 676:65–71
Becke AD (1988) Phys Rev A 38:3098–3100
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Hariharan PC, Pople JA (1973) Theor Chim Acta 28:213–222
McLean AD, Chandler GS (1980) J Chem Phys 72:5639–5648
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) J Chem Phys 72:650–654
Wachters AJH (1970) J Chem Phys 52:1033–1036
Hay PJ (1977) J Chem Phys 66:4377–4384
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03, Revision B.03. Gaussian Inc., Pittsburgh
Flükiger P, Lüthi HP, Portmann S, Weber J (2000) MOLEKEL 43. Swiss Center for Scientific Computing, Manno
Ochterski JW (2000) Thermochemistry in Gaussian. Gaussian Inc., Pittsburgh
Souza P, Sanz L, Fernandez V, Arquero A, Gutierrez E, Monge A (1991) Z Naturforsch Teil b 46:767–772
Acknowledgements
The authors are grateful for the partial support by the Thailand research Fund (TRF) and Rachadapisek Sompoch Endowment Fund, Chulalongkorn University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruangpornvisuti, V., Wanno, B. A DFT investigation of conformational geometries and interconversion equilibria of phenylthiosemicarbazone and its complexation with zinc. J Mol Model 10, 418–426 (2004). https://doi.org/10.1007/s00894-004-0217-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-004-0217-6