Abstract
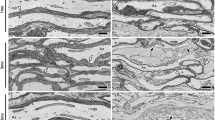
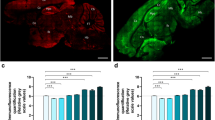
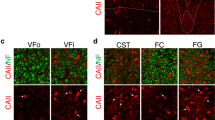
Impaired nerve conduction, axonal degeneration, and synaptic alterations contribute to neurological disabilities in inflammatory demyelinating diseases. Cerebellar dysfunction is associated with demyelinating disorders, but the alterations of axon terminals in cerebellar gray matter during chronic demyelination are still unclear. We analyzed the morphological and ultrastructural changes of climbing fiber terminals in a mouse model of hereditary chronic demyelination. Three-dimensional ultrastructural analyses using serial block-face scanning electron microscopy and immunostaining for synaptic markers were performed in a demyelination mouse model caused by extra copies of myelin gene (PLP4e). At 1 month old, many myelinated axons were observed in PLP4e and wild-type mice, but demyelinated axons and axons with abnormally thin myelin were prominent in PLP4e mice at 5 months old. The density of climbing fiber terminals was significantly reduced in PLP4e mice at 5 months old. Reconstruction of climbing fiber terminals revealed that PLP4e climbing fibers had increased varicosity volume and enlarged mitochondria in the varicosities at 5-month-old mice. These results suggest that chronic demyelination is associated with alterations and loss of climbing fiber terminals in the cerebellar cortex, and that synaptic changes may contribute to cerebellar phenotypes observed in hereditary demyelinating disorders.



Similar content being viewed by others
References
Dutta R, Ph D, Chomyk AM, Chang A, Ribaudo MV, Deckard SA, Doud MK, Edberg DD, Bai B, Li M, Baranzini SE, Fox RJ, Staugaitis SM, Macklin WB, Trapp BD (2013) Hippocampal demyelination and memory dysfunction are associated with increased levels of the neuronal microRNA miR-124 and reduced AMPA receptors. Ann Neurol 73:637–645. https://doi.org/10.1002/ana.23860.Hippocampal
Mandolesi G, Musella A, Gentile A, Grasselli G, Haji N, Sepman H, Fresegna D, Bullitta S, De Vito F, Musumeci G, Di Sanza C, Strata P, Centonze D (2013) Interleukin-1 alters glutamate transmission at Purkinje cell synapses in a mouse model of multiple sclerosis. J Neurosci 33:12105–12121. https://doi.org/10.1523/JNEUROSCI.5369-12.2013
Mandolesi G, Grasselli G, Musella A, Gentile A, Musumeci G, Sepman H, Haji N, Fresegna D, Bernardi G, Centonze D (2012) GABAergic signaling and connectivity on Purkinje cells are impaired in experimental autoimmune encephalomyelitis. Neurobiol Dis 46:414–424. https://doi.org/10.1016/j.nbd.2012.02.005
Watanabe M, Kano M (2011) Climbing fiber synapse elimination in cerebellar Purkinje cells. Eur J Neurosci 34:1697–1710. https://doi.org/10.1111/j.1460-9568.2011.07894.x
Hashimoto K, Ichikawa R, Kitamura K, Watanabe M, Kano M (2009) Translocation of a “winner” climbing fiber to the purkinje cell dendrite and subsequent elimination of “losers” from the soma in developing cerebellum. Neuron 63:106–118. https://doi.org/10.1016/j.neuron.2009.06.008
Xu-Friedman MA, Harris KM, Regehr WG (2001) Three-dimensional comparison of ultrastructural characteristics at depressing and facilitating synapses onto cerebellar Purkinje cells. J Neurosci 21:6666–6672
Kleim J, Freeman JH, Bruneau R, Nolan BC, Cooper NR, Zook A, Walters D (2002) Synapse formation is associated with memory storage in the cerebellum. Proc Natl Acad Sci USA 99:13228–13231. https://doi.org/10.1073/pnas.202483399
Kagawa T, Ikenaka K, Inoue Y, Kuriyama S, Tsujii T, Nakao J, Nakajima K, Aruga J, Okano H, Mikoshiba K (1994) Glial cell degeneration and hypomyelination caused by overexpression of myelin proteolipid protein gene. Neuron 13:427–442. https://doi.org/10.1016/0896-6273(94)90358-1
Nguyen HB, Thai TQ, Saitoh S, Wu B, Saitoh Y, Shimo S, Fujitani H, Otobe H, Ohno N (2016) Conductive resins improve charging and resolution of acquired images in electron microscopic volume imaging. Sci Rep 6:23721. https://doi.org/10.1038/srep23721
Thai TQ, Nguyen HB, Saitoh S, Wu B, Saitoh Y, Shimo S, Elewa YHA, Ichii O, Kon Y, Takaki T, Joh K, Ohno N (2016) Rapid specimen preparation to improve the throughput of electron microscopic volume imaging for three-dimensional analyses of subcellular ultrastructures with serial block-face scanning electron microscopy. Med Mol Morphol 49:154–162. https://doi.org/10.1007/s00795-016-0134-7
Cardona A, Saalfeld S, Schindelin J, Arganda-Carreras I, Preibisch S, Longair M, Tomancak P, Hartenstein V, Douglas RJ (2012) TrakEM2 software for neural circuit reconstruction. PLoS One 7:e38011. https://doi.org/10.1371/journal.pone.0038011
Hámori J, Szentágothai J (1966) Identification under the electron microscope of climbing fibers and their synaptic contacts. Exp Brain Res 1:65–81. https://doi.org/10.1007/BF00235210
Palay SL, Chan-Palay V (1974) Cerebellar cortex cytology and organization. Springer, Berlin, pp 253–285
Miyazaki T, Fukaya M, Shimizu H, Watanabe M (2003) Subtype switching of vesicular glutamate transporters at parallel fibre-Purkinje cell synapses in developing mouse cerebellum. Eur J Neurosci 17:2563–2572. https://doi.org/10.1046/j.1460-9568.2003.02698.x
Choo M, Miyazaki T, Yamazaki M, Kawamura M, Nakazawa T, Zhang J, Tanimura A, Uesaka N, Watanabe M, Sakimura K, Kano M (2017) Retrograde BDNF to TrkB signaling promotes synapse elimination in the developing cerebellum. Nat Commun 8:195. https://doi.org/10.1038/s41467-017-00260-w
Ma J, Tanaka KF, Shimizu T, Bernard CCA, Kakita A, Takahashi H, Pfeiffer SE, Ikenaka K (2011) Microglial cystatin F expression is a sensitive indicator for ongoing demyelination with concurrent remyelination. J Neurosci Res 89:639–649. https://doi.org/10.1002/jnr.22567
Willard HF, Riordan JR (1985) Assignment of the gene for myelin proteolipid protein to the X chromosome: implications for X-linked myelin disorders. Science 230:940–942. https://doi.org/10.1126/science.3840606
Saugier-Veber P, Munnich A, Bonneau D, Rozet J-M, Le Merrer M, Gil R, Boespflug-Tanguy O (1994) X-linked spastic paraplegia and Pelizaeus–Merzbacher disease are allelic disorders at the proteolipid protein locus. Nat Genet 6:257–262
Inoue K (2005) PLP1-related inherited dysmyelinating disorders: Pelizaeus–Merzbacher disease and spastic paraplegia type 2. Neurogenetics 6:1–16. https://doi.org/10.1007/s10048-004-0207-y
Garbern JY (2007) Pelizaeus–Merzbacher disease: genetic and cellular pathogenesis. Cell Mol Life Sci 64:50–65. https://doi.org/10.1007/s00018-006-6182-8
Readhead C, Schneider A, Griffiths I, Nave KA (1994) Premature arrest of myelin formation in transgenic mice with increased proteolipid protein gene dosage. Neuron 12:583–595. https://doi.org/10.1016/0896-6273(94)90214-3
Wolf NI, Sistermans EA, Cundall M, Hobson GM, Davis-Williams AP, Palmer R, Stubbs P, Davies S, Endziniene M, Wu Y, Chong WK, Malcolm S, Surtees R, Garbern JY, Woodward KJ (2005) Three or more copies of the proteolipid protein gene PLP1 cause severe Pelizaeus–Merzbacher disease. Brain 128:743–751. https://doi.org/10.1093/brain/awh409
Sarret C, Lemaire JJ, Tonduti D, Sontheimer A, Coste J, Pereira B, Feschet F, Roche B, Boespflug-Tanguy O (2016) Time-course of myelination and atrophy on cerebral imaging in 35 patients with PLP1-related disorders. Dev Med Child Neurol 58:706–713. https://doi.org/10.1111/dmcn.13025
Nevin ZS, Factor DC, Karl RT, Douvaras P, Laukka J, Windrem MS, Goldman SA, Fossati V, Hobson GM, Tesar PJ (2017) Modeling the mutational and phenotypic landscapes of Pelizaeus–Merzbacher disease with human iPSC-derived oligodendrocytes. Am J Hum Genet 100:617–634. https://doi.org/10.1016/j.ajhg.2017.03.005
Laukka JJ, Kamholz J, Bessert D, Skoff RP (2017) Novel pathologic findings in patients with Pelizaeus–Merzbacher disease. Neurosci Lett 627:222–232. https://doi.org/10.1016/j.neulet.2016.05.028
Koeppen AH, Robitaille Y (2002) Pelizaeus–Merzbacher disease. J Neuropathol Exp Neurol 61:747–759
Charzewska A, Wierzba J, Iżycka-Świeszewska E, Bekiesińska-Figatowska M, Jurek M, Gintowt A, Kłosowska A, Bal J, Hoffman-Zacharska D (2016) Hypomyelinating leukodystrophies—a molecular insight into the white matter pathology. Clin Genet 90:293–304. https://doi.org/10.1111/cge.12811
Seitelberger F (1995) Neuropathology and genetics of Pelizaeus–Merzbacher disease. Brain Pathol 5:267–273. https://doi.org/10.1111/j.1750-3639.1995.tb00603.x
Dutta R, Chang A, Doud MK, Kidd GJ, Ribaudo MV, Young EA, Fox RJ, Staugaitis SM, Trapp BD (2011) Demyelination causes synaptic alterations in hippocampi from multiple sclerosis patients. Ann Neurol 69:445–454. https://doi.org/10.1002/ana.22337
Jürgens T, Jafari M, Kreutzfeldt M, Bahn E, Brück W, Kerschensteiner M, Merkler D (2016) Reconstruction of single cortical projection neurons reveals primary spine loss in multiple sclerosis. Brain 139:39–46. https://doi.org/10.1093/brain/awv353
Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AMM, Lambris JD, Smith SJ, John SWM, Barres BA (2007) The classical complement cascade mediates CNS synapse elimination. Cell 131:1164–1178. https://doi.org/10.1016/j.cell.2007.10.036
Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT (2011) Development synaptic pruning by microglia is necessary for normal brain synaptic pruning by microglia is necessary for normal brain development. Science 333:1456–1459. https://doi.org/10.1126/science.1202529
Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, Smith SJ, Barres BA (2013) Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504:394–400. https://doi.org/10.1038/nature12776
Kakegawa W, Mitakidis N, Miura E, Abe M, Matsuda K, Takeo YH, Kohda K, Motohashi J, Takahashi A, Nagao S, Muramatsu SI, Watanabe M, Sakimura K, Aricescu AR, Yuzaki M (2015) Anterograde C1ql1 signaling is required in order to determine and maintain a single-winner climbing fiber in the mouse cerebellum. Neuron 85:316–330. https://doi.org/10.1016/j.neuron.2014.12.020
Nishiyama H, Fukaya M, Watanabe M, Linden DJ (2007) Axonal motility and its modulation by activity are branch-type specific in the intact adult cerebellum. Neuron 56:472–487. https://doi.org/10.1016/j.neuron.2007.09.010
Becker N, Wierenga CJ, Fonseca R, Bonhoeffer T, Nägerl UV (2008) LTD induction causes morphological changes of presynaptic boutons and reduces their contacts with spines. Neuron 60:590–597. https://doi.org/10.1016/j.neuron.2008.09.018
Felts PA, Kapoor R, Smith KJ (1995) A mechanism for ectopic firing in central demyelinated axons. Brain 118:1225–1231. https://doi.org/10.1093/brain/118.5.1225
Waxman SG (2008) Mechanisms of disease: sodium channels and neuroprotection in multiple sclerosis—current status. Nat Clin Pract Neurol 4:159–169. https://doi.org/10.1038/ncpneuro0735
Chavan V, Willis J, Walker SK, Clark HR, Liu X, Fox MA, Srivastava S, Mukherjee K (2015) Central presynaptic terminals are enriched in ATP but the majority lack mitochondria. PLoS One 10:1–19. https://doi.org/10.1371/journal.pone.0125185
Khatri N, Man HY (2013) Synaptic activity and bioenergy homeostasis: implications in brain trauma and neurodegenerative diseases. Front Neurol 4:1–11. https://doi.org/10.3389/fneur.2013.00199
Vercellino M, Merola A, Piacentino C, Votta B, Capello E, Mancardi GL, Mutani R, Giordana MT, Cavalla P (2007) Altered glutamate reuptake in relapsing-remitting and secondary progressive multiple sclerosis cortex: correlation with microglia infiltration, demyelination, and neuronal and synaptic damage. J Neuropathol Exp Neurol 66:732–739. https://doi.org/10.1097/nen.0b013e31812571b0
Matute C, Domercq M, Sánchez-Gómez M-V (2006) Glutamate-mediated glial injury: mechanisms and clinical importance. Glia 53:212–224. https://doi.org/10.1002/glia.20275
Sun T, Qiao H, Pan PY, Chen Y, Sheng ZH (2013) Motile axonal mitochondria contribute to the variability of presynaptic strength. Cell Rep 4:413–419. https://doi.org/10.1016/j.celrep.2013.06.040
MacAskill AF, Rinholm JE, Twelvetrees AE, Arancibia-Carcamo IL, Muir J, Fransson A, Aspenstrom P, Attwell D, Kittler JT (2009) Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron 61:541–555. https://doi.org/10.1016/j.neuron.2009.01.030
Billups B, Forsythe ID (2002) Presynaptic mitochondrial calcium sequestration influences transmission at mammalian central synapses. J Neurosci 22:5840–5847. https://doi.org/10.1523/JNEUROSCI.22-14-05840.2002
Acknowledgements
We thank Drs. M Yuzaki, A Kakegawa (Keio University) and M Watanabe (Hokkaido University) for helpful discussion and support. This work is partly supported by JSPS KAKENHI Grant number 16K12345 (to N.O.) and Grants-in-Aid for Scientific Research on Innovative Areas “glial assembly” (no. 25117005 to K.I.), and Research Grant from National Center of Neurology and Psychiatry (no. 30-5 to N.O.) and Novartis Pharma, Cooperative Research Program of “Network Joint Research Center for Materials and Devices” and Cooperative Study Programs of National Institute for Physiological Sciences (to N.O.). We would like to thank Setsuro Fujii Memorial, Osaka Foundation for Promotion of Fundamental Medical Research for providing the support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Nguyen, H.B., Sui, Y., Thai, T.Q. et al. Decreased number and increased volume with mitochondrial enlargement of cerebellar synaptic terminals in a mouse model of chronic demyelination. Med Mol Morphol 51, 208–216 (2018). https://doi.org/10.1007/s00795-018-0193-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-018-0193-z