Abstract
Although the understanding of the complex pathogenesis for osteoporosis is appreciable, the underlying mechanism is not yet fully elucidated. There is a great need to further characterize the available animal models in osteoporosis research. The senescence-accelerated mouse prone 6 (SAMP6) mice have been developed as the spontaneous experimental model for senile osteoporosis. Here, we provide a comprehensive overview of current research regarding the bone morphological and molecular alterations and the possible mechanisms involved in these changes. There were significant decrease in trabecular bone mass at the axial and appendicular skeletal sites, with no marked alterations of cortical bone. Decreased bone formation on the endosteal surface and trabecular bone, and increased bone marrow adiposity were observed in SAMP6 mice. The elevated expression level of proliferator activator gamma (PPARγ) in the bone marrow suggest that PPARγ might regulate osteoblastic bone formation negatively in SAMP6 mice. The expression level of secreted frizzled-related protein 4 (Sfrp4) was found to be higher in SAMP6 mice. Sfrp4 is considered to suppress osteoblastic proliferation mediated by inhibition of Wnt signaling pathway. These findings may help us to gain more insight into the potential mechanism of senile osteoporosis.

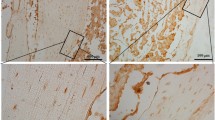
(modified from Ref. [21]). As compared with SAMR1, trabecular bone decreased and medullary cavity enlarged in SAMP6 mice. a, c, e SAMR1 mice; b, d, f SAMP6 mice. Scale bar 0.5 mm



Similar content being viewed by others
References
Chen H, Senda T, Kubo KY (2015) The osteocyte plays multiple roles in bone remodeling and mineral homeostasis. Med Mol Morphol 48:61–68
Chen H, Zhou X, Fujita H, Onozuka M, Kubo KY (2013) Age-related changes in trabecular and cortical bone microstructure. Int J Endocrinol 2013:213234
Kalu DN (1991) The ovariectomized rat model of postmenopausal bone loss. Bone Miner 15:175–191
Kalu DN, Chen C (1999) Ovariectomized murine model of postmenopausal calcium malabsorption. J Bone Miner Res 14:593–601
Kharode YP, Sharp MC, Bodine PV (2008) Utility of the ovariectomized rat as a model for human osteoporosis in drug discovery. Methods Mol Biol 455:111–124
Priemel M, Schilling AF, Haberland M, Pogoda P, Rueger JM, Amling M (2002) Osteopenic mice: animal models of the aging skeleton. J Musculoskelet Neuronal Interact 2:212–218
Watanabe K, Hishiya A (2005) Mouse models of senile osteoporosis. Mol Asp Med 26:221–231
Takeda T, Hosokawa M, Takeshita S, Irino M, Higuchi K, Matsushita T, Tomita Y, Yasuhira K, Hamamoto H, Shimizu K, Ishii M, Yamamuro T (1981) A new murine model of accelerated senescence. Mech Ageing Dev 17:183–194
Takeda T, Hosokawa M, Higuchi K (1991) Senescence-accelerated mouse (SAM): a new murine model of accelerated senescence. J Am Geriatr Soc 39:911–919
Takeda T, Matsushita Kurozumi M (1997) Pathobiology of the senescence-accelerated mouse (SAM). Exp Gerontol 32:117–127
Xia C, Higuchi K, Shimizu M et al (1999) Genetic typing of the senescence-accelerated mouse (SAM) strains with microsatellite markers. Mamm Genome 10:235–238
Matsushita M, Tsuboyama T, Kasai R, Okumura H, Yamamuro T, Higuchi K, Kohno A, Umezawa M, Takeda T (1986) Age-related changes in bone mass in the senescence-accelerated mouse (SAM): SAM-R/3 and SAM-P/6 as new murine models for senile osteoporosis. Am J Pathol 125:276–283
Jilka RL, Weinstein RS, Takahashi K, Parfitt AM, Manolagas SC (1996) Linkage of decreased bone mass with impaired osteoblastogenesis in a murine model of accelerated senescence. J Clin Invest 97:1732–1740
Chen H, Shoumura S, Emura S (2004) Ultrastructural changes in bones of the senescence-accelerated mouse (SAMP6): a murine model for senile osteoporosis. Histol Histopathol 19:677–685
Niimi K, Takahashi E, Itakura C (2009) Adiposity-related biochemical phenotype in senescence-accelerated mouse prone 6 (SAMP6). Comp Med 59:431–436
Liu CZ, Yu JC, Cheng HY, Jiang ZG, Li T, Zhang XZ, Zhang LL, Han JX (2006) Spatial memory performance and hippocampal neuron number in osteoporotic SAMP6 mice. Exp Neurol 201:452–460
Niimi K, Takahashi E, Itakura C (2008) Emotional behavior and expression patterns of tyrosine hydroxylase and tryptophan hydroxylase in senescence-accelerated mouse (SAM) P6 mice. Behav Brain Res 188:329–336
Chen H, Emura S, Yao XF, Shoumura S (2004) Morphological study of the parathyroid gland and thyroid C cell in senescence-accelerated mouse (SAMP6), a murine model for senile osteoporosis. Tissue Cell 36:409–415
Chen H, Emura S, Shoumura S (2006) Ultrastructure of the water-clear cell in the parathyroid gland of SAMP6 mice. Tissue Cell 38:187–192
Chen H, Yao XF, Emura S, Shoumura S (2006) Morphological changes of skeletal muscle, tendon and periosteum in the senescence-accelerated mouse (SAMP6): a murine model for senile osteoporosis. Tissue Cell 38:325–335
Chen H, Zhou X, Emura S, Shoumura S (2009) Site-specific bone loss in senescence-accelerated mouse (SAMP6): a murine model for senile osteoporosis. Exp Gerontol 44:792–798
Chen H, Kubo KY (2012) Segmental variations in trabecular bone density and microstructure of the spine in senescence-accelerated mouse (SAMP6): a murine model for senile osteoporosis. Exp Gerontol 47:317–322
Chen H, Emura S, Isono H, Shoumura S (2005) Effects of traditional Chinese medicine on bone loss in SAMP6: a murine model for senile osteoporosis. Biol Pharm Bull 28:865–869
Silva MJ, Brodt MD, Wopenka B, Thomopoulos S, Williams D, Wassen MH, Ko M, Kusano N, Bank RA (2006) Decreased collagen organization and content are associated with reduced strength of demineralized and intact bone in the SAMP6 mouse. J Bone Miner Res 21:78–88
Silva MJ, Brodt MD, Ko M, Abu-Amer Y (2005) Impaired marrow osteogenesis is associated with reduced endocortical bone formation but does not impair periosteal bone formation in long bones of SAMP6 mice. J Bone Miner Res 20:419–427
Tokutomi K, Matsuura T, Atsawasuwan P, Sato H, Yamauchi M (2008) Characterization of mandibular bones in senile osteoporotic mice. Connect Tissue Res 49:361–366
Ganguly P, El-Jawhari JJ, Giannoudis PV, Burska AN, Ponchel F, Jones EA (2017) Age-related changes in bone marrow mesenchymal stromal cells: a potential impact on osteoporosis and osteoarthritis development. Cell Transpl 26:1520–1529
Bethel M, Chitteti BR, Srour EF, Kacena MA (2013) The changing balance between osteoblastogenesis and adipogenesis in aging and its impact on hematopoiesis. Curr Osteoporos Rep 11:99–106
Liu H, Xia X, Li B (2015) Mesenchymal stem cell aging: mechanisms and influences on skeletal and non-skeletal tissues. Exp Biol Med (Maywood) 240:1099–1106
Ichioka N, Inaba M, Kushida T, Esumi T, Takahara K, Inaba K, Ogawa R, Iida H, Ikehara S (2002) Prevention of senile osteoporosis in SAMP6 mice by intrabone marrow injection of allogeneic bone marrow cells. Stem Cells 20:542–551
Takada K, Inaba M, Ichioka N, Ueda Y, Taira M, Baba S, Mizokami T, Wang X, Hisha H, Iida H, Ikehara S (2006) Treatment of senile osteoporosis in SAMP6 mice by intra-bone marrow injection of allogeneic bone marrow cells. Stem Cells 24:399–405
Ueda Y, Inaba M, Takada K, Fukui J, Sakaguchi Y, Tsuda M, Omae M, Kushida T, Iida H, Ikehara S (2007) Induction of senile osteoporosis in normal mice by intra-bone marrow-bone marrow transplantation from osteoporosis-prone mice. Stem Cells 25:1356–1363
Kajkenova O, Lecka-Czernik B, Gubrij I, Hauser SP, Takahashi K, Parfitt AM, Jilka RL, Manolagas SC, Lipschitz DA (1997) Increased adipogenesis and myelopoiesis in the bone marrow of SAMP6, a murine model of defective osteoblastogenesis and low turnover osteopenia. J Bone Miner Res 12:1772–1779
Sui B, Hu C, Liao L, Chen Y, Zhang X, Fu X, Zheng C, Li M, Wu L, Zhao X, Jin Y (2016) Mesenchymal progenitors in osteopenias of diverse pathologies: differential characteristics in the common shift from osteoblastogenesis to adipogenesis. Sci Rep 6:30186
Cao J, Ou G, Yang N, Ding K, Kream BE, Hamrick MW, Isales CM, Shi XM (2015) Impact of targeted PPARγ disruption on bone remodeling. Mol Cell Endocrinol 410:27–34
Sun H, Kim JK, Mortensen R, Mutyaba LP, Hankenson KD, Krebsbach PH (2013) Osteoblast-targeted suppression of PPARγ increases osteogenesis through activation of mTOR signaling. Stem Cells 31:2183–2192
Meunier P, Aaron J, Edouard C, Vignon G (1971) Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res 80:147–154
Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM (1999) PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 4:611–617
Tsuboyama T, Takahashi K, Matsushita M, Okumura H, Yamamuro T, Umezawa M, Takeda T (1989) Decreased endosteal formation during cortical bone modelling in SAM-P/6 mice with a low peak bone mass. Bone Miner 7:1–12
Tsuboyama T, Takahashi K, Yamamuro T, Hosokawa M, Takeda T (1993) Cross-mating study on bone mass in the spontaneously osteoporotic mouse (SAM-P/6). Bone Miner 23:57–64
Shimizu M, Higuchi K, Bennett B, Xia C, Tsuboyama T, Kasai S, Chiba T, Fujisawa H, Kogishi K, Kitado H, Kimoto M, Takeda N, Matsushita M, Okumura H, Serikawa T, Nakamura T, Johnson TE, Hosokawa M (1999) Identification of peak bone mass QTL in a spontaneously osteoporotic mouse strain. Mamm Genome 10:81–87
Otsuki B, Matsumura T, Shimizu M, Mori M, Okudaira S, Nakanishi R, Higuchi K, Hosokawa M, Tsuboyama T, Nakamura T (2007) Quantitative trait locus that determines the cross-sectional shape of the femur in SAMP6 and SAMP2 mice. J Bone Miner Res 22:675–685
Okudaira S, Shimizu M, Otsuki B, Nakanishi R, Ohta A, Higuchi K, Hosokawa M, Tsuboyama T, Nakamura T (2010) Quantitative trait locus on chromosome X affects bone loss after maturation in mice. J Bone Miner Metab 28:520–531
Nakanishi R, Shimizu M, Mori M, Akiyama H, Okudaira S, Otsuki B, Hashimoto M, Higuchi K, Hosokawa M, Tsuboyama T, Nakamura T (2006) Secreted frizzled-related protein 4 is a negative regulator of peak BMD in SAMP6 mice. J Bone Miner Res 21:1713–1721
Nakanishi R, Akiyama H, Kimura H, Otsuki B, Shimizu M, Tsuboyama T, Nakamura T (2008) Osteoblast-targeted expression of Sfrp4 in mice results in low bone mass. J Bone Miner Res 23:271–277
Haraguchi R, Kitazawa R, Mori K, Tachibana R, Kiyonari H, Imai Y, Abe T, Kitazawa S (2016) sFRP4-dependent Wnt signal modulation is critical for bone remodeling during postnatal development and age-related bone loss. Sci Rep 6:25198
Lee DY, Kim H, Ku SY, Kim SH, Choi YM, Kim JG (2010) Association between polymorphisms in Wnt signaling pathway genes and bone mineral density in postmenopausal Korean women. Menopause 17:1064–1070
Fujita M, Urano T, Shiraki M, Momoeda M, Tsutsumi O, Hosoi T, Orimo H, Ouchi Y, Inoue S (2004) Association of a single nucleotide polymorphism in the secreted frizzled-related protein 4 (sFRP4) gene with bone mineral density. Geriatr Gerontol Int 4:175–180
Clément-Lacroix P, Ai M, Morvan F, Roman-Roman S, Vayssière B, Belleville C, Estrera K, Warman ML, Baron R, Rawadi G (2005) Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci USA 102:17406–17411
Chen X, Li L, Guo J, Zhang L, Yuan Y, Chen B, Sun Z, Xu J, Zou J (2016) Treadmill running exercise prevents senile osteoporosis and upregulates the Wnt signaling pathway in SAMP6 mice. Oncotarget 7:71072–71086
Kramer I, Halleux C, Keller H, Pegurri M, Gooi JH, Weber PB, Feng JQ, Bonewald LF, Kneissel M (2010) Osteocyte Wnt/β-catenin signaling is required for normal bone homeostasis. Mol Cell Biol 30:3071–3085
MacDonald BT, Tamai K, He X (2009) Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell 17:9–26
Baron R, Kneissel M (2013) Wnt signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19:179–192
Beighton P (1987) Pyle disease (metaphyseal dysplasia. J Med Genet 24:321–324
Galada C, Shah H, Shukla A, Girisha KM (2017) A novel sequence variant in SFRP4 causing Pyle disease. J Hum Genet 62:575–576
Kiper POS, Saito H, Gori F, Unger S, Hesse E, Yamana K, Kiviranta R, Solban N, Liu J, Brommage R, Boduroglu K, Bonafé L, Campos-Xavier B, Dikoglu E, Eastell R, Gossiel F, Harshman K, Nishimura G, Girisha KM, Stevenson BJ, Takita H, Rivolta C, Superti-Furga A, Baron R (2016) Cortical-bone fragility—insights from sFRP4 deficiency in Pyle’s disease. N Engl J Med 374:2553–2562
Chen H, Zhou X, Shoumura S, Emura S, Bunai Y (2010) Age- and gender-dependent changes in three-dimensional microstructure of cortical and trabecular bone at the human femoral neck. Osteoporos Int 21:627–636
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Azuma, K., Zhou, Q. & Kubo, Ky. Morphological and molecular characterization of the senile osteoporosis in senescence-accelerated mouse prone 6 (SAMP6). Med Mol Morphol 51, 139–146 (2018). https://doi.org/10.1007/s00795-018-0188-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-018-0188-9