Abstract
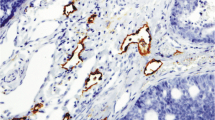
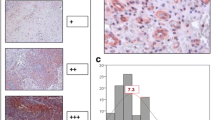
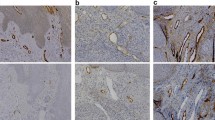
Lymphatic dissemination is one of the most important pathways for metastasis in many solid tumors, including head and neck carcinomas. The lymphatic growth of cancer has been used as a significant independent adverse prognostic factor and provides information about tumor progression. Salivary gland tumors present different prognoses and have the ability to develop metastases; however, this information regarding the lymphatic spread is scarce. This paper quantifies the lymphatic microvessel density (LMD) in benign and malignant salivary gland tumors and analyzes the relationship between LMD and tumor expression of vascular endothelial growth factors C (VEGF-C) and the proliferative index. The results show that there is no correlation between LMD, VEGF-C and the proliferative index in the majority of salivary gland tumors analyzed, apart from polymorphous low-grade carcinoma which exhibits statistical correlation between LMD and the proliferative index (p < 0.05). This correlation probably does not indicate a poor prognosis for this PLGA, since this is a low metastasizing carcinoma of the salivary glands. Different from other solid tumors, such as breast or prostatic carcinomas, there is no correlation between VEGF-C and LMD in salivary gland tumors, and so these traits are not able to estimate the metastatic risk or the prognosis of these tumors.


Similar content being viewed by others
References
Barnes L, Eveson JW, Reichart P, Sidransky D (2005) Tumors of the salivary glands. In: Barnes L, Eveson JW, Reichart P, Sidransky D (eds) World Health Organization classification of tumors. Pathology and genetics head and neck tumors. IARC Press, Lyon, pp 209–281
Ito FA, Ito K, Vargas PA, de Almeida OP, Lopes MA (2005) Salivary gland tumors in a Brazilian population: a retrospective study of 496 cases. Int J Oral Maxillofac Surg 34:533–536
Tian Z, Li L, Wang L, Hu Y, Li J (2010) Salivary gland neoplasms in oral and maxillofacial regions: a 23-year retrospective study of 6982 cases in an eastern Chinese population. Int J Oral Maxillofac Surg 39:235–242
Loyola AM, Araújo VC, Sousa SO, Araújo VC (1995) Minor salivary gland tumors: a retrospective study of 164 cases in Brazilian population. Eur J Cancer B Oral Oncol 31B:197–201
Yap TA, Lorente D, Omlin A, Olmos D, de Bono JS (2014) Circulating tumor cells: a multifunctional biomarker. Clin Cancer Res 20:2553–2568
Hansen S, Grabaud DA, Sorensen FB, Bak M, Vach W, Rose C (2000) The prognostic value of angiogenesis by chalkley counting in a confirmatory study design on breast cancer patients. Clin Cancer Res 6:139–146
Han Z, Chen Z, Zheng R, Cheng Z, Wang D (2015) Clinicopathological significance of CD133 and CD44 expression in infiltrating ductal carcinoma and their relationship to angiogenesis. World J Surg Oncol 13:486
Matilla MMT, Ruohola JK, Karpanen T, Jackson DG, Alitalo K, Härkönen P (2002) VEGF-C induced lymphangiogenesis is associated with lymph node metastasis in orthotopic MCF-7 tumors. Int J Cancer 98:946–951
Dadras SS, Lange-Asschenfeldt B, Velasco P, Nguyen L, Vora A, Muzi Kansky A, Jahnke K, Hauschild A, Hirakawa S, Mihm MC, Detmar M (2005) Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. Mod Pathol 18:1232–1242
Gombos Z, Xu X, Chu CS, Zhang PJ, Acs G (2005) Peritumoral lymphatic vessel density and vascular endothelial growth factor C expression in early stage squamous cell carcinoma of the uterine cervix. Clin Cancer Res 11:8364–8371
Franchi A, Gallo O, Massi D, Baroni G, Santucci M (2004) Tumor lymphangiogenesis in head and neck squamous cell carcinoma: a morphometric study with clinical correlations. Cancer 101:973–978
Takes RP (2010) Staging of the neck in patients with head and neck squamous cell cancer: imaging techniques and biomarkers. Oral Oncol 40:656–667
Zhang Z, Helman JI, Li LJ (2010) Lymphangiogenesis, lymphatic endothelial cells and lymphatic metastasis in head and neck cancer—a review of mechanisms. Int J Oral Sci 2:5–14
Nobis CP, Rohleder NH, Wolff KD, Wagenpfeil S, Scherer EQ, Kesting MR (2014) Head and neck salivary gland carcinomas–elective neck dissection, yes or no? J Oral Maxillofac Surg 72:205–210
Herman MP, Werning JW, Morris CG, Kirwan JM, Amdur RJ, Mendenhall WM (2013) Elective neck management for high-grade salivary gland carcinoma. Am J Otolaryngol 34:205–208
Szöke T, Kayser K, Baumhäkel JD, Trojan I, Furak J, Tiszlavicz L, Eller J, Boda K (2005) Prognostic significance of microvascularization in cases of operated lung cancer. Eur J Cardiothorac Surg 27:1106–1111
Schipper RJ, Moossdorff M, Nelemans PJ, Nieuwenhuijzen GA, de Vries B, Strobbe LJ et al (2014) A model to predict pathologic complete response of axillary lymph nodes to neoadjuvant chemo(immuno)therapy in patients with clinically node-positive breast cancer. Clin Breast Cancer 14:315–322
Emmett MS, Lanati S, Dunn DB, Stone OA, Bates DO (2011) CCR7 mediates directed growth of melanomas towards lymphatics. Microcirculation. 18:172–182
Joyce JA, Pollard JW (2009) Microenvironmental regulation of metastasis. Nat Rev Cancer 9:239–252
Spinella F, Garrafa E, Di Castro V, Rosanò L, Nicotra MR, Caruso A et al (2009) Endothelin-1 stimulates lymphatic endothelial cells and lymphatic vessels to grow and invade. Cancer Res 69:2669–2676
Achen MG, Stacker SA (2008) Molecular control of lymphatic metastasis. Ann N Y Acad Sci 1131:225–234
Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K (2002) Lymphangiogenesis and cancer metastasis. Nat Rev Cancer 2:573–583
Evangelou E, Kyzas PA, Trikalinos TA (2005) Comparison of the diagnostic accuracy of lymphatic endothelium markers: bayesian approach. Mod Pathol 18:1490–1497
Dang Q, Liu J, Li J, Sun Y (2014) Podoplanin: a novel regulator of tumor invasion and metastasis. Med Oncol 9:24
Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA (2010) Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 11:174–183
Zhu X, Zhang J, Chen X, Feng X (2012) Comparison of Ki-67, cyclin E, and p63 in benign and malignant human pleomorphic adenoma. Oral Surg Oral Med Oral Pathol Oral Radiol 113:667–672
Ben-Izhak O, Akrish S, Nagler RM (2008) Ki67 and salivary cancer. Cancer Invest 26:1015–1023
Scholzen T, Gerdes J (2000) The Ki-67 protein: from the known and the unknown. J Cell Phisiol. 182:311–322
Karatzanis AD, Koudounarakis E, Papadakis I, Velegrakis G (2012) Molecular pathways of lymphangiogenesis and lymph node metastasis in head and neck cancer. Eur Arch Otorhinolaryngol 269:731–737
Cheuk W, Chan JKC (2007) Advances in salivary gland pathology. Histopathology 51:1–20
Duff SE, Jeziorska M, Kumar S, Haboubi N, Sherlock D, O’Dwyer ST, Jayson GC (2007) Lymphatic vessel density, microvessel density and lymphangiogenic growth factor expression in colorectal cancer. Colorectal Dis 9:793–800
Raica M, Cimpean AM, Ceausu R, Ribatti D (2011) Lymphatic microvessel density, VEGF-C, and VEGFR-3 expression in different molecular types of breast cancer. Anticancer Res 31:1757–1764
Kostis G, Ioannis L, Helen K, Helen P (2014) The expression of vascular endothelial growth factor-C correlates with lymphatic microvessel density and lymph node metastasis in prostate carcinoma: an immunohistochemical study. Urol Ann. 6:224–230
Choi WW, Lewis MM, Lawson D, Yin-Goen Q, Birdsong GG, Cotsonis GA et al (2005) Angiogenic and lymphangiogenic microvessel density in breast carcinoma: correlation with parameters and VEGF-family gene expression. Mod Pathol 18:143–152
Fujita G, Sato S, Kishino M, Iwai S, Nakazawa M, Toyosawa S, Yura Y, Ogawa Y (2011) Lymphatic vessels and related factors in adenoid cystic carcinoma of the salivary gland. Mod Pathol 24:885–891
Gleber-Netto FO, Florêncio TN, de Sousa SF, Abreu MH, Mendonça EF, Aguiar MC (2012) Angiogenesis and lymphangiogenesis in mucoepidermoid carcinoma of minor salivary glands. J Oral Pathol Med 41:603–609
Mello MF, Costa AP, Freitas LL, Soares AB, Araujo VC, Tincani AJ, Martins AS, Altemani A (2011) Lymphatic vessel density and expressions of lymphangiogenic growth factors in salivary carcinomas. Neoplasma. 58:331–336
R, Stárek I, Kučerová L, Skálová A, Hoza J (2014) Neither expression of VEGF-C/D nor lymph vessel density supports lymphatic invasion as the mechanism responsible for local spread of recurrent salivary pleomorphic adenoma. Virchows Arch 464:29–34
Karaman S, Hollmén M, Robciuc MR, Alitalo A, Nurmi H, Morf B, Buschle D, Alkan HF, Ochsenbein AM, Alitalo K, Wolfrum C, Detmar M (2014) Blockade of VEGF-C and VEGF-D modulates adipose tissue inflammation and improves metabolic parameters under high-fat diet. Mol Metab 4:93–105
Salzman R, Stárek I, Kučerová L, Skálová A, Hoza J (2014) Neither expression of VEGF-C/D nor lymph vessel density supports lymphatic invasion as the mechanism responsible for local spread of recurrent salivary pleomorphic adenoma. Virchows Arch 464:29–34
Tampouris AI, Kandiloros D, Giotakis I, Gakiopoulou H, Lazaris AC (2012) The role of the VEGF-C/-D/flt-4 autocrine loop in the pathogenesis of salivary neoplasms. Pathol Res Pract 208:151–156
Stárek I, Salzman R, Kučerová L, Skálová A, Hauer L (2015) Expression of VEGF-C/-D and lymphangiogenesis in salivary adenoid cystic carcinoma. Pathol Res Pract 211:759–765
Yoo SH, Roh JL, Kim SO, Cho KJ, Choi SH, Nam SY, Kim SY (2015) Patterns and treatment of neck metastases in patients with salivary gland cancers. J Surg Oncol 111:1000–1006
Ghazy SE, Helmy IM, Baghdadi HM (2011) Maspin and MCM2 immunoprofiling in salivary gland carcinomas. Diagn Pathol 6:89
Patel TD, Vazquez A, Marchiano E, Park RC, Baredes S, Eloy JA (2015) Polymorphous low-grade adenocarcinoma of the head and neck: a population-based study of 460 cases. Laryngoscope 125:1644–1649
Acknowledgments
Coordination for the Improvement of Higher Education Personnel (CAPES).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Americo, M.G., Marques, Y.M.F.S., El Abras Ankha, M.D.V. et al. Correlation of intratumoral lymphatic microvessel density, vascular endothelial growth factor C and cell proliferation in salivary gland tumors. Med Mol Morphol 50, 17–24 (2017). https://doi.org/10.1007/s00795-016-0142-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-016-0142-7