N
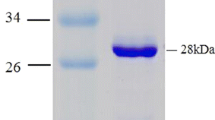
5,N 10-Methenyltetrahydromethanopterin cyclohydrolase (Mch) is an enzyme involved in methanogenesis from CO2 and H2 which represents the energy metabolism of Methanopyrus kandleri, a methanogenic Archaeon growing at a temperature optimum of 98°C. The gene mch from M. kandleri was cloned, sequenced, and expressed in Escherichia coli. The overproduced enzyme could be purified in yields above 90% in one step by chromatography on phenyl Sepharose in 80% ammonium sulfate. From 3.5 g cells (250 mg protein), approximately 18 mg cyclohydrolase was obtained. The purified enzyme showed essentially the same catalytic properties as the enzyme purified from M. kandleri cells. The primary structure and properties of the cyclohydrolase are compared with those of the enzyme from Methanococcus jannaschii (growth temperature optimum 85°C), from Methanobacterium thermoautotrophicum (65°C), and from Methanosarcina barkeri (37°C). Of the four enzymes, that from M. kandleri has the lowest isoelectric point (3.8) and the lowest hydrophobicity of amino acid composition. Besides, it has the highest relative content of glutamate, leucine, and valine and the lowest relative content of isoleucine, serine, and lysine. Some of these properties are unusual for enzymes from hyperthermophilic organisms. They may reflect the observation that the cyclohydrolase from M. kandleri is not only adapted to hyperthermophilic conditions but also to the high intracellular concentrations of lyotrophic salts prevailing in this organism.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: July 14, 1997 / Accepted: August 28, 1997
Rights and permissions
About this article
Cite this article
Vaupel, M., Vorholt, J. & Thauer, R. Overproduction and one-step purification of the N 5,N 10-methenyltetrahydro-methanopterin cyclohydrolase (Mch) from the hyperthermophilic Methanopyrus kandleri . Extremophiles 2, 15–22 (1998). https://doi.org/10.1007/s007920050038
Issue Date:
DOI: https://doi.org/10.1007/s007920050038