Abstract
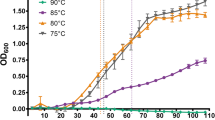
When non-extremophiles encounter extreme environmental conditions, which are natural for the extremophiles, stress reactions, e.g., expression of heat shock proteins (HSPs), are thought to be induced for survival. To understand how the extremophiles live in such extreme environments, we studied the effects of high hydrostatic pressure on cellular contents of HSPs and their mRNAs during growth in a piezophilic bacterium, Shewanella violacea. HSPs increased at high hydrostatic pressures even when optimal for growth. The mRNAs and proteins of these HSPs significantly increased at higher hydrostatic pressure in S. violacea. In the non-piezophilic Escherichia coli, however, their mRNAs decreased, while their proteins did not change. Several transcriptional start sites (TSSs) for HSP genes were determined by the primer extension method and some of them showed hydrostatic pressure-dependent increase of the mRNAs. A major refolding target of one of the HSPs, chaperonin, at high hydrostatic pressure was shown to be RplB, a subunit of the 50S ribosome. These results suggested that in S. violacea, HSPs play essential roles, e.g., maintaining protein complex machinery including ribosomes, in the growth and viability at high hydrostatic pressure, and that, in their expression, the transcription is under the control of σ32.








Similar content being viewed by others
References
Aertsen A, Vanoirbeek K, De Spiegeleer P, Sermon J, Hauben K, Farewell A, Nystrom T, Michiels CW (2004) Heat shock protein-mediated resistance to high hydrostatic pressure in Escherichia coli. Appl Environ Microbiol 70:2660–2666
Alix JH, Nierhaus KH (2003) DnaK-facilitated ribosome assembly in Escherichia coli revisited. RNA 9:787–793
Allen EE, Bartlett DH (2002) Piezophiles: microbial adaptation to the deep-sea environment. extremophiles III. In: Gerday C, Glandorff N (eds) Encyclopedia of life support system. Eolss Publishers, Oxford, pp 231–255
Aono E, Baba T, Ara T, Nishi T, Nakamichi T, Inamoto E, Toyonaga H, Hasegawa M, Takai Y, Okumura Y, Baba M, Tomita M, Kato C, Oshima T, Nakasone K, Mori H (2010) Complete genome sequence and comparative analysis of Shewanella violacea, a psychrophilic and piezophilic bacterium from deep sea floor sediments. Mol BioSyst 6:1216–1226
Arsene F, Tomoyasu T, Bukau B (2000) The heat shock response of Escherichia coli. Int J Food Microbiol 55:3–9
Bartlett DH (1999) Microbial adaptations to the psychrosphere/piezosphere. J Mol Microbiol Biotechnol 1:93–100
Black SL, Dawson A, Ward FB, Allen RJ (2013) Genes required for growth at high hydrostatic pressure in Escherichia coli K-12 identified by genome-wide screening. PLoS One 8:e73995. doi:10.1371/jounal.pone.0073995
Blaszczak A, Georgopoulos C, Liberek K (1999) On the mechanism of FtsH-dependent degradation of the sigma 32 transcriptional regulator of Escherichia coli and the role of the Dnak chaperone machine. Mol Microbiol 31:157–166
Bukau B, Horwich AL (1998) The Hsp70 and Hsp60 chaperone machines. Cell 92:351–366
Cowing DW, Bardwell JC, Craig EA, Woolford C, Hendrix RW, Gross CA (1985) Consensus sequence for Escherichia coli heat shock gene promoters. Proc Natl Acad Sci USA 82:2679–2683
El Hage A, Sbaï M, Alix JH (2001) The chaperonin GroEL and other heat-shock proteins, besides DnaK, participate in ribosome biogenesis in Escherichia coli. Mol Gen Genet 264:796–808
Ewalt KL, Hendrick JP, Houry WA, Hartl FU (1997) In vivo observation of polypeptide flux through the bacterial chaperonin system. Cell 90:491–500
Fenton WA, Horwich AL (2003) Chaperonin-mediated protein folding: fate of substrate polypeptide. Q Rev Biophys 36:229–256
Gruber TM, Gross CA (2003) Multiple sigma subunits and the partitioning of bacterial transcription space. Ann Rev Microbiol 57:441–466
Guisbert E, Yura T, Rhodius VA, Gross CA (2008) Convergence of molecular, modeling, and systems approaches for an understanding of the Escherichia coli heat shock response. Microbiol Mol Biol Rev 72:545–554
Hartl FU (1996) Molecular chaperones in cellular protein folding. Nature 381:571–579
Hasan CM, Shimizu K (2008) Effect of temperature up-shift on fermentation and metabolic characteristics in view of gene expressions in Escherichia coli. Microb Cell Fact 7:35. doi:10.1186/1475-2859-7-35
Hawley DK, McClure WR (1983) Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res 11:2237–2255
Houry WA, Frishman D, Eckerskorn C, Lottspeich F, Hartl FU (1999) Identification of in vivo substrates of the chaperonin GroEL. Nature 402:147–154
Hummer G, Garde S, Garcia AE, Paulaitis ME, Pratt LR (1998) The pressure dependence of hydrophobic interactions is consistent with the observed pressure denaturation of proteins. Proc Natl Acad Sci USA 95:1552–1555
Infante AA, Demple B, Chaires JB (1982) Analysis of the Escherichia coli ribosome-ribosomal subunit equilibrium using pressure-induced dissociation. J Biol Chem 257:80–87
Ishii A, Oshima T, Sato T, Nakasone K, Mori H, Kato C (2005) Analysis of hydrostatic pressure effects on transcription in Escherichia coli by DNA microarray procedure. Extremophiles 9:65–73
Jenkins DE, Auger EA, Matin A (1991) Role of RpoH, a heat shock regulator protein, in Escherichia coli carbon starvation protein synthesis and survival. J Bacteriol 173:1992–1996
Kanemori M, Nishihara K, Yanagi H, Yura T (1997) Synergistic roles of HslVU and other ATP-dependent proteases in controlling in vivo turnover of σ32 and abnormal proteins in Escherichia coli. J Bacteriol 179:7219–7225
Kato C (2006) Handling of piezophilic microorganisms. In: Rainey F, Oren A (eds) Extremophiles, a volume in the methods in microbiology series, vol 35. Academic Press, Elsevier, pp 733–741
Kato C, Qureshi MH (1999) Pressure response in deep-sea piezophilic bacteria. J Mol Microbiol Biotechnol 1:87–92
Kato C, Sato T, Horikoshi K (1995) Isolation and properties of barophilic and barotolerant bacteria from deep-sea mud samples. Biodivers Conserv 4:1–9
Kato C, Sato T, Nakasone K, Tamegai H (2008) High-Pressure Microbiology. In: Michiels C, Bartlett DH, Aertsen A (ed), ASM Press, Washington, pp 305–317
Lindquist S, Craig EA (1988) The heat-shock proteins. Ann Rev Genet 22:631–677
Lipinska B, King J, Ang D, Georgopoulos C (1988) Sequence analysis and transcriptional regulation of the Escherichia coli grpE gene, encoding a heat shock protein. Nucleic Acids Res 16:7545–7562
Lowry OH, Rosebrough NJ, Farr AL, Randal RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Marquis RE (1976) High-pressure microbial physiology. Adv Microb Physiol 14:159–241
Miller AD, Maghlaoui K, Albanese G, Kleinjan DA, Smith C (1993) Escherichia coli chaperonins cpn60 (groEL) and cpn10 (groES) do not catalyse the refolding of mitochondrial malate dehydrogenase. Biochem J 291:139–144
Mosteller RD, Goldstein RV (1975) Unusual sensitivity of Escherichia coli to adenine or adenine plus histidine. J Bacteriol 123:750–751
Murata M, Fujimoto H, Nishimura K, Charoensuk K, Nagamitsu H, Raina S, Kosaka T, Oshima T, Ogasawara N, Yamada M (2011) Molecular strategy for survival at a critical high temperature in Escherichia coli. PLoS One 6:e20063
Nagai H, Yano R, Erickson JW, Yura T (1990) Transcriptional regulation of the heat shock regulatory gene rpoH in Escherichia coli: involvement of a novel catabolite-sensitive promoter. J Bacteriol 172:2710–2715
Nakasone K, Ikegami A, Kato C, Horikoshi K (2001) Isolation of the rpoD gene encoding the principal sigma factor of the deep-sea piezophilic bacterium Shewanella violacea strain DSS12 and its overexpression in Escherichia coli. Biosci Biotechnol Biochem 65:190–193
Narberhaus F (1999) Negative regulation of bacterial heat shock genes. Mol Microbiol 31:1–8
Niven GW, Miles CA, Mackey BM (1999) The effects of hydrostatic pressure on ribosome conformation in Escherichia coli: and in vivo study using differential scanning calorimetry. Microbiology 145:419–425
Nogi Y, Kato C, Horikoshi K (1998) Taxonomic studies of deep-sea barophilic Shewanella strains and description of Shewanella violacea sp. nov. Arch Microbiol 170:331–338
Ohmae E, Miyashita Y, Kato C (2013) Thermodynamic and functional characteristics of deep-sea enzymes revealed by pressure effects. Extremophiles 17:701–709
Parsell DA, Lindquist S (1993) The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Ann Rev Genet 27:437–496
Richter K, Haslbeck M, Buchner J (2010) The heat shock response: life on the verge of death. Mol Cell 40:253–266
Romero DP, Arredondo JA, Traut RR (1990) Identification of a region of Escherichia coli ribosomal protein L2 required for the assembly of L16 into the 50 S ribosomal subunit. J Biol Chem 265:18185–18191
Rudolph B, Gebendorfer KM (2010) Evolution of Escherichia coli for growth at high temperatures. J Biol Chem 285:19029–19034
Siegenthaler RK, Christen P (2005) The importance of having thermosensor control in the DnaK chaperone system. J Biol Chem 280:14395–14401
Straus DB, Walter WA, Gross CA (1987) The heat shock response of E. coli is regulated by changes in the concentration of σ32. Nature 329:348–351
Straus D, Walter W, Gross CA (1990) DnaK, DnaJ, and GrpE heat shock proteins negatively regulate heat shock gene expression by controlling the synthesis and stability of σ32. Genes Dev 4:2202–2209
Tomoyasu T, Gamer J, Bukau B, Kanemori M, Mori H, Rutman AJ, Oppenheim AB, Yura T, Yamanaka K, Niki H (1995) Escherichia coli FtsH is a membrane-bound, ATP-dependent protease which degrades the heat-shock transcription factor σ32. EMBO J 14:2551–2560
Tosco A, Birolo L, Madonna S, Lolli G, Sannia G, Marino G (2003) GroEL from the psychrophilic bacterium Pseudoalteromonas haloplanktis TAC 125: molecular characterization and gene cloning. Extremophiles 7:17–28
Weber G, Drickamer HG (1983) The effect of high pressure upon proteins and other biomolecules. Q Rev Biophys 16:89–112
Welch TJ, Farewell A, Neidhardt FC, Bartlett DH (1993) Stress response of Escherichia coli to elevated hydrostatic pressure. J Bacteriol 175:7170–7177
Yamauchi S, Okuyama H, Morita EH, Hayashi H (2003) Gene structure and transcriptional regulation specific to the groESL operon from the psychrophilic bacterium Colwellia maris. Arch Microbiol 180:272–278
Yayanos AA (1995) Microbiology to 10,500 meters in the deep sea. Ann Rev Microbiol 49:777–805
Yayanos AA, Pollard EC (1969) A study of the effects of hydrostatic pressure on macromolecular synthesis in Escherichia coli. Biophys J 9:1464–1482
Yura T, Nakahigashi K (1999) Regulation of the heat-shock response. Curr Opin Microbiol 2:153–158
Yura T, Kawasaki Y, Kusukawa N, Nagai H, Wada C, Yano R (1990) Roles and regulation of the heat shock sigma factor σ32 in Escherichia coli. Antonie Van Leeuwenhoek 58:187–190
Zhao K, Liu M, Burgess RR (2005) The global transcriptional response of Escherichia coli to induced sigma 32 protein involves sigma 32 regulon activation followed by inactivation and degradation of sigma 32 in vivo. J Biol Chem 280:17758–17768
Zobell CE, Cobet AB (1962) Growth, reproduction, and death rates of Escherichia coli at increased hydrostatic pressures. J Bacteriol 84:1228–1236
Zobell CE, Cobet AB (1964) Filament formation by Escherichia coli at increased hydrostatic pressures. J Bacteriol 87:710–719
Zuber U, Schumann W (1994) CIRCE, a novel heat shock element involved in regulation of heat shock operon dnaK of Bacillus subtilis. J Bacteriol 176:1359–1363
Acknowledgments
This study was supported by a grant from the 21st Century COE Program, Ministry of Education, Culture, Sports, Science and Technology. We thank A. Ishihama (Nippon Institute for Biological Science) for providing the σ32 antibody, and A. Nishimura and T. Uehara (Department of Bioscience and Biotechnology, Aomori University) for a gift of the HscA antibody. We also thank Dr. H. Kuramitsu for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Robb.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sato, H., Nakasone, K., Yoshida, T. et al. Increases of heat shock proteins and their mRNAs at high hydrostatic pressure in a deep-sea piezophilic bacterium, Shewanella violacea . Extremophiles 19, 751–762 (2015). https://doi.org/10.1007/s00792-015-0751-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-015-0751-4