Abstract
Planococcus halocryophilus OR1 is a bacterial isolate capable of growth at temperatures ranging from −15 to +37 °C. During sub-zero (cryophilic) growth, nodular features appear on its cell surface; however, the biochemical compositions of these features as well as any cold-adaptive benefits they may offer are not understood. This study aimed to identify differences in the cell surface proteome (surfaceome) of P. halocryophilus cells grown under optimal (24 °C, no added salt), low- and mid-salt (5 and 12 % NaCl, respectively) at 24 °C, and low- and mid-salt sub-zero (5 % NaCl at −5 °C and 12 % NaCl at −10 °C) culture conditions, for the purpose of gaining insight into cold-adapted proteomic traits at the cell surface. Mid-log cells were harvested, treated briefly with trypsin and the resultant peptides were purified followed by identification by LC–MS/MS analysis. One hundred and forty-four proteins were subsequently identified in at least one culture condition. Statistically significant differences in amino acid usage, a known indicator of cold adaptation, were identified through in silico analysis. Two proteins with roles in peptidoglycan (PG) metabolism, an N-acetyl-l-alanine amidase and a multimodular transpeptidase–transglycosylase, were detected, though each was only detected under optimal conditions, indicating that high-salt and high-cold stress each affect PG metabolism. Two iron transport-binding proteins, associated with two different iron transport strategies, were identified, indicating that P. halocryophilus uses a different iron acquisition strategy at very low temperatures. Here we present the first set of data that describes bacterial adaptations at the cellular surface that occur as a cryophilic bacterium is transitioned from optimal to near-inhibitory sub-zero culture conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Planococcus halocryophilus OR1 is a Gram-positive bacteria isolated from active-layer soil overlaying permafrost in the Canadian High Arctic near Eureka, NU (Mykytczuk et al. 2012). It is capable of growth and cellular division between +37 and −15 °C—which is the coldest cellular replication temperature reported to date (Mykytczuk et al. 2013)—and metabolic activity continues to at least −25 °C (Mykytczuk et al. 2013). P. halocryophilus is also halophilic and able to grow in up to 18.5 % NaCl (Mykytczuk et al. 2012). The combination of low-temperature and high-salt tolerance is likely an evolutionary requirement since in situ sub-zero growth of P. halocryophilus likely takes place in brine veins that form on soil particles as solutes are excluded from forming ice crystals (Gilichinsky et al. 1993; Rivkina et al. 2000; Jakosky et al. 2003). P. halocryophilus cells grown at optimal temperature (24 °C) have a smooth surface with occasional nodular surface features that occur along the division planes. However, cells grown at −15 °C are thickly encrusted in a nodular sheet-like crust, which may result from peptidoglycan accumulating at division planes and precipitating nano-crystalline minerals from the growth media (Mykytczuk et al. 2013). These visible alterations on the cell surface of P. halocryophilus, in response to cryophilic growth, may be adaptive and provide some level of protection against freezing, although possible mechanisms are not understood. Defining the cell surface proteome (surfaceome) of P. halocryophilus both at optimal and sub-zero culture temperatures may provide insight into adaptive cell surface changes in response to cryophilic growth.
Cold growth has severe physiochemical consequences for bacteria. Cell integrity, water viscosity, diffusion rates, membrane fluidity, and enzyme kinetics are all negatively affected and psychrophilic, psychrotrophic, and cryophilic bacteria must have several adaptive strategies to maintain vital cell functions at low temperatures (De Maayer et al. 2014). To maintain protein function, cold-adapted proteins have increased flexibility, decreased thermostability, and increased specific activity (De Maayer et al. 2014). To satisfy these criteria, proteins have cold-adapted amino acid substitutions that lead to decreased arginine:lysine ratios, proline, acidic and aliphatic residues, and hydrophobicity compared to mesophilic equivalents (Ayala-del-Río et al. 2010; De Maayer et al. 2014a). Most cold-adapted bacteria also contain isozymes, multiple forms of the same proteins with the same function but composed of different amino acids, of essential proteins (Mykytczuk et al. 2013). P. halocryophilus has a total of 302 genes which have one or more (up to 15) isozymes encoded within its genome (Mykytczuk et al. 2013).
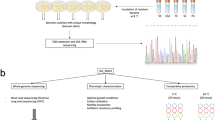
Bacterial surface-exposed proteins are at the frontline of interactions between the cell and its environment. For environmental isolates, surface-associated proteins are responsible for substrate attachment, cell-to-cell communication, quorum sensing, nutrient uptake, and waste secretion making them critically important in the success of a bacterial strain in a specific environmental niche (Tjalsma et al. 2008; Cao and Bazemore-Walker 2014). However, the major focus of bacterial surfaceomics has been on medically relevant isolates since during infection these proteins interact with host cells and are the most promising vaccine candidates (Rodríguez-Ortega et al. 2006; Severin et al. 2007; Tjalsma et al. 2008; Benachour et al. 2009; Bøhle et al. 2011; Berlec et al. 2011; Olaya-Abril et al. 2014). Using techniques recently pioneered to examine the bacterial surfaceome (Solis et al. 2010) the aim of this study was to define the surfaceome of P. halocryophilus OR1 during growth at 24 °C in TSB, 24 °C in increased salt media (5 and 12 % NaCl), and sub-zero temperatures (−5 and −10 °C) to provide insight into how the cell surface adjusts to sub-zero growth as well as the nature of the surface-associated nodular features observed during cryophilic growth (Mykytczuk et al. 2013).
Methods and materials
Bacterial strains and culture conditions
P. halocryophilus OR1 (Mykytczuk et al. 2013) stock cultures were maintained in tryptic soy broth (TSB) at 24 °C. Cells used for surfaceomics were grown in each of five different culture conditions: at 24 °C in TSB (optimal conditions), at 24 °C in low-salt media (LSM) (TSB supplemented with 5 % NaCl and 2 % glycerol), at 24 °C in medium-salt media (MSM) (TSB supplemented with 12 % NaCl and 5 % glycerol), at −5 °C in LSM, and at −10 °C in MSM. Salts were added to the sub-zero media in these concentrations to prevent media from freezing solid at sub-zero temperatures. Cultures grown at 24 °C with additional salts were included in the study to allow meaningful comparisons to be made between proteomic changes occurring due to salt stress versus those occurring due to temperature stress. All LSM cultures were inoculated with P. halocryophilus grown to mid-log phase (26 h) in TSB at 24 °C, at a 1:10 inoculum to LSM media ratio. MSM cultures were inoculated with 1:5 inoculum to media ratio. Cells grown for surfaceomic analysis were grown without agitation and harvested during mid-log phase; though, dependent on growth conditions mid-log was reached at different time points. Cells grown at 24 °C in TSB were harvested after 26 h, after 10 days in LSM, or after 33 days in MSM. Cells grown at −5 °C in LSM were harvested after 67 days of growth and cells grown at −10 °C in MSM were harvested after 123 days of growth. Cell density at mid-log phase also varied depending upon growth conditions and, therefore, different volumes were required to obtain the average of 1 × 1010 cells, used for each surface protein extraction. For cells grown at 24 °C in TSB, at 24 °C in LSM and at −5 °C in LSM 50 mL of culture was used for each surface protein extraction. The volume of culture used was increased to 150 mL for cells grown in MSM at −10 °C and to 300 mL for cells grown at 24 °C in MSM.
Cell surface peptide purification
Surface-associated peptides were purified using a modified version of the Solis et al. protocol (Solis et al. 2010). Briefly, mid-log cells were separated from media by centrifugation at 3000×g for 15 min, washed three times in ice-cold wash solution (20 mM Tris–HCl, 150 mM NaCl, pH 7.6), and suspended in 2 mL re-suspension buffer (20 mM Tris–HCl, 150 mM NaCl, 10 mM CaCl2, 0.6 M sucrose, pH 7.6). Experiments were carried out in biological triplicate. Each suspension was incubated at 37 ℃ with 5 μg (2.5 μg mL−1) sequencing grade modified trypsin for 15 min (Promega, Madison, WI, USA). Whole cells were separated from peptides in solution by centrifugation at room temperature, for 1 min at 14,000×g, and the supernatant was passed through a sterile 0.24-μm filter (Fisher, Rockford, IL, USA). Negative controls were washed and incubated using the same procedure but without the addition of enzyme then separated from intact cells by centrifugation, and the supernatant was passed through a filter. Negative control supernatant was digested by 5 μg of trypsin at 37 °C for 15 min. All peptides were purified with Pierce C-18 Spin Columns (Fisher) as per the manufacturer’s instructions. Purified peptides were dried gently using a vacuum evaporator.
LC–MS/MS Sample preparations
Peptides were desalted and purified using C18 Ziptips (Millipore, Billerica, MA, USA) according to the manufacturer’s instructions. Samples were then evaporated to dryness in a vacuum centrifuge, resuspended in 20 μL of injection solvent (3 % acetonitrile, 0.2 % formic acid, and 0.05 % trifluoroacetic acid [TFA] (v/v) in water) for LC–MS/MS analysis.
Mass spectrometric (MS) analysis
The Q-TOF instrument (Waters Synapt HDMS QTOF) was calibrated by infusion prior to analysis with Glu-Fibrinopetide (100 fmol μL−1). For each sample, triplicate 2 μL aliquots were analyzed by loading onto a Waters Symmetry C18 trap column (180 μm × 20 mM with 5 μm particles) and washing with 0.1 % formic acid in water (solvent A) for 3 min at 5.0 μL min−1 before separating on a Waters nanoAcquity UPLC BEH130 C18 reverse-phase analytical column (100 μm × 100 mm with 1.7 μm particles). Chromatographic separation was achieved at a flow rate of 0.5 μL min−1 over 70 min in six linear steps as follows (solvent B was 0.1 % formic acid in acetonitrile): initial, 3 % B; 2 min, 10 % B; 40 min, 30 % B; 50 min, 95 % B; 55 min, 95 % B; 56 min, 3 % B; end, 3 % B. MS and LC–MS/MS analyzed the eluted peptides in data directed analysis (DDA) mode. MS survey scans were 1 s in duration, and LC–MS/MS data were collected on the top four most abundant peaks until either the total ion count exceeded 4000 or 3 s elapsed. All peaks selected for LC–MS/MS analysis during the first analysis of a particular sample were used to generate an exclusion list for the second analysis, and this was reiterated for the third analysis.
MS data processing
DDA data were processed using Mascot software (Matrix Science). The raw data were processed using Mascot Distiller (version 2.4.2.0) to create Mascot generic files (MGFs) which were submitted for database searching using Mascot Daemon (version 2.4.0), and database searches were performed using Mascot (version 2.4), against the NCBI database (downloaded April 16, 2014) specifying taxonomy P. halocryophilus OR1. A search against a decoy database was performed to obtain an estimation of false discovery rates. Peptide and LC–MS/MS mass tolerances were 100 ppm and 0.1 Da, respectively, and tryptic peptides having charges from 2+ to 4+ and up to two missed cleavages were considered. Carbamidomethyl derivatization of cysteine was set as fixed modification, and oxidation of methionine and deamidation of asparagine and glutamine were specified as variable modifications. Mascot search result files (DATs) and PLGS results files were loaded into Scaffold (Proteome Science, version 4.3.2) for visualization.
Analysis of cold adaptation
The protein sequences that were identified by Mascot were analyzed for amino acid cold-adaptive features including the number of proline residues, the arginine:lysine ratio, hydrophobicity, the aliphatic index, aromaticity, and the number of acidic residues. These cold adaptation features were measured for each protein identified in each of the five conditions: 24 °C in LSM media, 24 °C in MSM media, −5 °C in LSM media, −10 °C in MSM media, and 24 °C in TSB media, then an average determined for each condition using the ProtParam (Wilkins et al. 1999) module of Biopython (Cock et al. 2009) and an in-house python script. The analysis was not limited to the proteins expressed exclusively at a given condition, but rather was conducted on the complete set of proteins detected in each of the conditions, regardless of whether they were present across multiple conditions or exclusive to a given condition. An unpaired t test was used to evaluate if there were statistically significant differences (p < 0.05) between each of the salt- and cold-stress growth conditions when compared to the optimally grown culture (24 °C in TSB).
Bioinformatic sub-cellular localization predictions
Proteins identified by Mascot were analyzed for sub-cellular localization and signal peptide presence by PSORTb v.3.0.2 (Yu et al. 2010). Signal peptide presence was also examined with SignalP v.4.1 (Petersen et al. 2011) using default settings, except D-cutoff value, which was set to sensitive.
Results and discussion
Bacterial surface-associated proteins are difficult to study since they have low abundance relative to cytoplasmic proteins, low solubility, and it is difficult to isolate pure surface fractions without cytoplasmic contamination (Solis and Cordwell 2011). First-generation, gel-based proteomics generally under-represents cell surface proteins since low solubility causes them to precipitate during electrophoresis (Olaya-Abril et al. 2014). Second-generation, gel-free approaches use shaving technique, also used in this investigation, that selectively digests protein fragments present on the cell surface; then the resultant peptides can then be identified by LC–MS/MS analysis. This is considered the “true surfaceomics approach” (Cordwell 2006). The surface shaving method used in this investigation yielded a semi-quantitative inventory of proteins that are present on the bacterial cell surface. Proteins that were not detected with Mascot scores >67 in at least two of three biological replicates, for each growth condition, were removed from further analysis. This resulted in a dataset containing 144 unique proteins, identified in at least one of the five investigated culture conditions. From this data set, 24 proteins were identified in all five conditions, 7 were uniquely identified in cultures grown at 24 °C in TSB, 19 were in all culture conditions except for 24 °C in TSB, 22 proteins were unique to −5 °C cultures, 1 was uniquely found in −10 °C cultures. Two proteins were found in both −5 °C and −10 °C cultures (Fig. 1a). Detailed results have been deposited into the ProteomeXchange database under the accession number PXD001083.
Venn diagram showing proteins identified as overlapping between various culture conditions. Each of the five culture conditions investigated here had proteins that were unique to that culture condition, though, more frequently proteins overlapped with at least one other condition. The numbers of proteins identified in each of the five culture conditions as well as all overlapping combinations are displayed for a all proteins identified in this investigation and b proteins that were not predicted to be primarily localized in the cytoplasm
PSORTb analysis predicted that signal peptides were present in 12 (8 %) of the identified proteins and SignalP predicted signal peptides in 13 proteins; each signal peptide predicted by PSORTb was also predicted by SignalP (Table 1). PSORTb was also used to divide identified proteins into five groups based on predicted cellular localization: 109 (75.7 %) proteins were cytoplasmic, 21 (14.5 %) had unknown localization, 10 (6.9 %) were bound to the cytoplasmic membrane, 3 (2.0 %) were anchored in the cell wall, and 1 (0.7 %) was secreted into the extracellular milieu (Table 1).
Protein adaptations to sub-zero growth
To retain functional activity at low temperature, cold-adapted proteins generally exhibit greater flexibility than mesophilic equivalents due to changes in amino acid usage (Bakermans et al. 2009). Cold-adapted modifications reduce the number of residues involved in hydrogen bonding and salt bridges, lower core hydrophobicity, and decrease residues that cause protein rigidity. The total set of proteins identified in each of the salt- and cold-stress culture conditions was compared to the set of proteins identified from cells grown at 24 °C in TSB to determine any possible changes in proline content, hydrophobicity, aromaticity, arginine/lysine ratio, acidic residues and aliphatic index. Average values were determined for the presence of each of these traits in the proteins at a given condition (Table 2). Proteins identified in the −5 °C LSM cultures, as well as the two 24 °C salt-stressed cultures, had significantly lower (p < 0.05) hydrophobicity (Table 2). Aromaticity was found to be significantly lower in the 24 °C LSM and −10 °C MSM cultures, and marginally lower (p < 1) in the 24 °C MSM culture. Less acidic and proline residues were found in the 24 °C LSM and −10 °C MSM culture conditions; however, these changes were not observed in the 24 °C MSM or −5 °C LSM cultures. Interestingly, a significantly higher arginine/lysine ratio was found in every non-optimal condition. Proteins with a significantly different aliphatic index were not identified in any of the culture conditions.
Together, these results point to a significant level of cold adaptation present in proteins identified from P. halocryophilus when grown in salt- or cold-stress conditions, which are generally consistent with modifications observed in other cryophiles (Arpigny et al. 1997; Methé et al. 2005; Riley et al. 2008; Ayala-del-Río et al. 2010) and from the genomic analysis of P. halocryophilus (Mykytczuk et al. 2013). Changes in the abundance of acidic and proline residues are only statistically significant at −10 °C but not −5 °C, therefore, it is possible that these adaptations (abundance of acidic and proline residues) become more important at lower temperatures. Higher arginine/lysine ratios observed in all non-optimal conditions are surprising and inconsistent with findings in other cold adaptive studies, which have often found a reduced arginine content in cold-adapted proteins (De Maayer et al. 2014). One possible explanation for this observation is that in addition to being cold adapted, Planococcus halocryophilus shows evidence of being ‘hot adapted’ (Mykytczuk et al. 2013). In addition, Mykytczuk et al. (2013) did not find a lower arginine/lysine ratio to be a significant indicator of cold adaptation at the genome-wide level. However, this does not address why the ratio would be higher in the sub-zero and salt-stressed culture conditions when compared to optimal. It is possible that the higher ratio observed in this study points to a hitherto unknown role of arginine in cold and/or salt adaptation which would be, to date, unique to P. halocryophilus.
Predicted cytoplasmic proteins
Although this study employed the false-positive strategy to reduce the number of cytoplasmic proteins (Solis et al. 2010), a high-percentage of predicted cytoplasmic proteins (75.7 %) were still identified. This percentage is high, although, comparable to additional studies that used a similar surface shaving technique, where 6–70 % of the observed surfaceome is bioinformatically predicted to be primarily cytoplasmic localized (Tjalsma et al. 2008; Solis et al. 2010; Bøhle et al. 2011; Zhang et al. 2013; Olaya-Abril et al. 2014). There are several explanations for the high number of predicted cytoplasmic proteins that remain in the final surfaceome dataset including bioinformatic misidentification of cellular localization, the presence of moonlighting proteins, and cellular lysis (Jeffery 2005; Solis and Cordwell 2011).
Bioinformatic techniques can be used to predict surface-exposed proteins from whole cell proteomic experiments or to verify the results of gel-free surface shaving. Although, different algorithms employ various methods to search for certain protein features, in general, surface-associated proteins are identified by calculating hydrophobicity in helical stretches, identifying surface anchoring domains, and searching for the presence of secretion or signal sequences (Solis and Cordwell 2011). Therefore, cell surface proteins that are anchorless and have no signal mechanism present a problem for algorithms and are generally identified as being cytoplasmic (Chhatwal 2002; Glowalla et al. 2009; Solis and Cordwell 2011). Some true surface-associated proteins identified in this investigation were likely bioinformatically identified as cytoplasmic proteins for some of these reasons.
Moonlighting refers to multifunctional proteins where two or more discrete functions are performed by the same unmodified peptide chain and appears to be common (Jeffery 2005). Several cytoplasmic proteins have been shown to have alternate roles at the cell surface (Jeffery 2005). For example, in Streptococcus pyogenes, proteins from the glycolytic pathway moonlight on the bacterial surface where they have adhesive properties (Henderson and Martin 2013). Ribosomal proteins are consistently found on the cell surface after the shaving approach and could have alternative moonlighting roles (Severin et al. 2007; Tjalsma et al. 2008); however, alternate explanations could also contribute. Pyruvate dehydrogenase (PDH) E1 (gi| 495773484), a heterodimer (alpha- and beta- units), is part of a complex that catalyzes the conversion of pyruvate to acetyl-CoA and CO2, and is essential in cellular metabolism was also identified. There have been previous observations of PDH in complex with membrane-bound ribosomal protein (MBRP) at the cellular membrane (Hemilä et al. 1990). The abundance of ribosomal protein detected on the cell surface in this study, may be explained by ribosomal proteins closely associated with PDH at the cell membrane during trypsin digestion; the digestion of PDH could result in a hole in the membrane allowing trypsin to digest PDH-associated ribosomal proteins in large enough abundances to be detected during analysis. Though this is speculation, it would provide an explanation of these results; however, we did not provide direct evidence for this.
Alternatively, these cytoplasmic proteins may have been identified due to contamination from cell lysis or were proteins released by shedding the membrane–vesicle structures in which they were trapped during trypsin digestion (Olaya-Abril et al. 2014). Since protein moonlighting roles at the cell surface are not clearly defined, and the primary objective of this work was to identify the functional sub-zero surfaceome, proteins predicted by PSORTb to be primarily cytoplasmic were not included in our analysis of the bacterial surface activity (Figs. 1b, 2).
Cell growth and division
Bacterial cell division is directed by the divisome, a multi-protein complex, which includes essential and non-essential proteins—FtsZ is foremost among the essential proteins (de Boer et al. 1992; Meier and Goley 2014). The presence of FtsZ has been detected on the cell surface in other surface shaving studies where cells were taken from mid-log phase (Zhang et al. 2013). Here we demonstrate the presence of FtsZ (gi| 495773432) in each of the tested growth conditions, indicating cells are undergoing active cellular division at the time of protein shaving (Garrido et al. 1993) (Fig. 2). FtsH is a cytoplasmic membrane protease with roles in cell division in several Gram-positive bacteria including Bacillus subtilis and Staphylococcus aureus (Lin et al. 2014). Our detection of FtsH in all growth conditions—excluding optimal growth conditions—corresponds nicely with its previously documented role in stress response, most notably to high-salt conditions (Fischer et al. 2002; Lithgow et al. 2004).
As cells grow and divide the peptidoglycan sacculi undergoes constant remodeling to allow for cellular expansion and in the final stages of cellular division peptidoglycan must be hydrolyzed to allow for daughter cell separation. Only two proteins with dedicated roles in peptidoglycan metabolism [N-acetylmuramoyl-l-alanine amidase (gi| 495772357) and multimodular transpeptidase-transglycosylase (gi| 495773182)] were detected in this study, and these were only detected in optimally grown cells. Peptidoglycan is composed of alternating N-acetylglucosamine and N-acetylmuramic acid residues, cross-linked by short peptide bridges. In the final stages of peptidoglycan synthesis, disaccharide units are polymerized by transglycosylase and peptide chains are cross-linked by transpeptidase (Ramachandran et al. 2006). The absence of the multimodular transpeptidase–transglycosylase in salt- and cold-stressed cells may indicate lower levels of active peptidoglycan synthesis in these conditions. N-acetylmuramoyl-l-alanine amidase is a peptidoglycan hydrolase responsible for hydrolyzing peptidoglycan at the glycan–peptide interface. In the absence of a functioning hydrolase, peptidoglycan may accumulate at the cells surface during remodeling, sacculi expansion, and cell division resulting in the nodular surface structures observed in previous work (Mykytczuk et al. 2013).
Cell membrane structure
Preservation of cell membrane fluidity during cold temperature growth is necessary for continued viability. In response to cold temperatures membrane fluidity is generally maintained by changing lipid composition favoring shorter chains and decreased lipid saturation (De Maayer et al. 2014). Our observed presence, of fatty-acid desaturase (gi| 495774159) in cold stressed but not in optimally grown cells, therefore, was expected. However, P. halocryophilus cells at sub-zero temperatures have been previously assessed for fatty-acid saturation and it was shown that, surprisingly, saturation increases with decreasing temperature (Mykytczuk et al. 2013). This indicates that P. halocryophilus employs an alternate mechanism for preserving membrane fluidity. Combined these results may indicate that the fatty-acid desaturases are present but inactive at these temperatures.
Transport systems
Compatible solutes can be important cryo/osmoprotectants in cryophilic and halophilic organisms. Glycine betaine ABC transport system-binding protein (OpuAC) (gi| 495772060) was uniquely identified in this study in −5 °C cultures. However, only one significant sequence (Table 1) was used to identify OpuAC, indicating relatively low abundance. This agrees with previous work, which has indicated that the Opu system is not up-regulated during mid-log phase in response to cold or salt stress, since osmotic balance is reached quickly at early log phase and these systems are no longer required beyond a short up-regulation during initial osmotic upshift (Mykytczuk et al. 2013). In a related microorganism, Bacillus subtilis, the glycine betaine transport system was responsible for the accumulation of several compatible solutes including: l-carnitine, crotonobetaine, butyrobetaine, homobetaine, dimethylsulfonioactetate, and proline betaine (Hoffmann and Bremer 2011). Our group is currently working on elucidating which solutes are imported via this pathway, during sub-zero growth.
Iron is essential for nearly all life. Two different ferric iron ABC transport-binding proteins were identified on the cell surface of P. halocryophilus. The first (gi| 495774171) was only present in optimal conditions, and has been shown to be associated with siderophore-mediated iron uptake in some species (Marchler-Bauer et al. 2013), while the second: ferric iron ABC transporter (gi|495773646) was identified in each culture condition. This may suggest that although P. halocryophilus is capable of both siderophore-mediated and ABC-type iron acquisition, siderophore-mediated iron uptake is not used during times of salt- or cold stress. This finding corroborates other work, on Antarctic bacteria, that has shown that siderophore-mediated iron uptake may not be the optimal mechanism during growth in stressful conditions (Pakchung et al. 2008).
Energetics
Two isoforms of the ATP synthase beta chain, involved in coupling ATP synthesis with the transport of protons across the cell membrane (essential for energetics), gi| 495772323 and gi| 495772326, were identified as part of the surfaceome in this investigation. The former was only identified in −10 °C cultures, while the latter was identified in all conditions excluding optimal, though, peptides matching gi| 495772326 were identified twofold more often in −10 °C grown cells relative to other conditions. This implies greater ATP synthesis is correlated with cryophilic conditions, which is consistent with previous proteomic work since a greater amount of energy is required per unit of biomass during low-temperature growth (Bakermans and Nealson 2004; Mykytczuk et al. 2013).
Concluding remarks
This study is the first to examine how the surfaceome of an environmental isolate shifts as the bacterium is transitioned from optimal to sub-zero growth temperatures. The results presented here, combined with earlier studies (Mykytczuk et al. 2013), indicate that halophilic and cryophilic adaptations in P. halocryophilus are intimately linked at the proteomic level and cold-adapted proteins tend to be identified during both sub-zero and optimal-temperature salt-stressed culture conditions. Given the environmental context, in which P. halocryophilus likely lives in brine veins during periods of freezing (Gilichinsky et al. 1993; Rivkina et al. 2000; Jakosky et al. 2003), this finding is reasonable from an environmental perspective.
Beyond the implications of this study in basic science to determine how the bacterial surface adapts to cryophilic conditions, further investigation of the surfaceomics of cryophilic bacteria may have broader implications in vaccine development (Duplantis et al. 2010) and geomicrobiology (Ronholm et al. 2014). The surfaceomics approach outlined in the current study has helped to identify deficiencies in peptidoglycan metabolism that occur in high-salt and low-temperature conditions and may be at least partially responsible for the unique nodular features previously observed on P. halocryophilus cells cultured in sub-zero conditions, other causes of these features and any adaptive roles they may play must be further investigated.
References
Arpigny JL, Lamotte J, Gerday C (1997) Molecular adaptation to cold of an Antarctic bacterial lipase. J Mol Catal 3:29–35. doi:10.1016/S1381-1177(96)00041-0
Ayala-del-Río HL, Chain PS, Grzymski JJ et al (2010) The genome sequence of Psychrobacter arcticus 273-4, a psychroactive Siberian permafrost bacterium, reveals mechanisms for adaptation to low-temperature growth. Appl Environ Microbiol 76:2304–2312. doi:10.1128/AEM.02101-09
Bakermans C, Nealson KH (2004) Relationship of critical temperature to macromolecular synthesis and growth yield in Psychrobacter cryopegella. J Bacteriol 186:2340–2345. doi:10.1128/JB.186.8.2340-2345.2004
Bakermans C, Bergholz PW, Ayala-del-Río H, Tiedje J (2009) Genomic insights into cold adaptation of permafrost bacteria. Permafrost soils. Springer, Berlin Heidelberg, pp 159–168
Benachour A, Morin T, Hébert L et al (2009) Identification of secreted and surface proteins from Enterococcus faecalis. Can J Microbiol 55:967–974. doi:10.1139/w09-052
Berlec A, Zadravec P, Jevnikar Z, Štrukelj B (2011) Identification of candidate carrier proteins for surface display on Lactococcus lactis by theoretical and experimental analyses of the surface proteome. Appl Environ Microbiol 77:1292–1300. doi:10.1128/AEM.02102-10
Bøhle LA, Riaz T, Egge-Jacobsen W et al (2011) Identification of surface proteins in Enterococcus faecalis V583. BMC Genom 12:135. doi:10.1186/1471-2164-12-135
Cao Y, Bazemore-Walker CR (2014) Science direct. J Proteomics 97:187–194. doi:10.1016/j.jprot.2013.08.011
Chhatwal GS (2002) Anchorless adhesins and invasins of Gram-positive bacteria: a new class of virulence factors. Trends Microbiol 10:205–208. doi:10.1016/S0966-842X(02)02351-X
Cock PJA, Antao T, Chang JT et al (2009) Biopython: freely available Python tools for computational molecular biology and bioinformatics. J Gerontol 25:1422–1423. doi:10.1093/bioinformatics/btp163
Cordwell SJ (2006) Technologies for bacterial surface proteomics. Curr Opin Microbiol 9:320–329. doi:10.1016/j.mib.2006.04.008
de Boer P, Crossley R, Rothfield L (1992) The essential bacterial cell-division protein FtsZ is a GTPase. Nature 359:254–256. doi:10.1038/359254a0
De Maayer P, Anderson D, Cary C, Cowan DA (2014) Some like it cold: understanding the survival strategies of psychrophiles. EMBO Rep 15:508–517. doi:10.1002/embr.201338170
Duplantis BN, Osusky M, Schmerk CL et al (2010) Essential genes from Arctic bacteria used to construct stable, temperature-sensitive bacterial vaccines. PNAS 107:13456–13460. doi:10.1073/pnas.1004119107
Fischer B, Rummel G, Aldridge P, Jenal U (2002) The FtsH protease is involved in development, stress response and heat shock control in Caulobacter crescentus. Mol Microbiol 44:461–478. doi:10.1046/j.1365-2958.2002.02887.x
Garrido T, Sanchez M, Palacios P, Aldea M (1993) Transcription of ftsZ oscillates during the cell cycle of Escherichia coli. EMBO J 12(10):3958–3965
Gilichinsky DA, Soina VS, Petrova MA (1993) Cryoprotective properties of water in the Earth cryolithosphere and its role in exobiology. Orig Life Evol Biosph 23:65–75
Glowalla E, Tosetti B, Kronke M, Krut O (2009) Proteomics-based identification of anchorless cell wall proteins as vaccine candidates against Staphylococcus aureus. Infect Immun 77:2719–2729. doi:10.1128/IAI.00617-08
Hemilä H, Palva A, Paulin L et al (1990) Secretory S complex of Bacillus subtilis: sequence analysis and identity to pyruvate dehydrogenase. J Bacteriol 172:5052–5063
Henderson B, Martin A (2013) Bacterial moonlighting proteins and bacterial virulence. Between pathogenicity and commensalism. Springer, Berlin Heidelberg, pp 155–213
Hoffmann T, Bremer E (2011) Protection of Bacillus subtilis against cold stress via compatible-solute acquisition. J Bacteriol 193:1552–1562. doi:10.1128/JB.01319-10
Jakosky BM, Nealson KH, Bakermans C et al (2003) Subfreezing activity of microorganisms and the potential habitability of Mars’ polar regions. Astrobiology 3:343–350. doi:10.1089/153110703769016433
Jeffery CJ (2005) Mass spectrometry and the search for moonlighting proteins. Mass Spectrom Rev 24:772–782. doi:10.1002/mas.20041
Lin T-H, Hu Y-N, Shaw G-C (2014) Two enzymes, TilS and HprT, can form a complex to function as a transcriptional activator for the cell division protease gene ftsH in Bacillus subtilis. J Biochem 155:5–16. doi:10.1093/jb/mvt081
Lithgow JK, Ingham E, Foster SJ (2004) Role of the hprT-ftsH locus in Staphylococcus aureus. Microbiology 150:373–381. doi:10.1099/mic.0.26674-0
Marchler-Bauer A, Zheng C, Chitsaz F et al (2013) CDD: conserved domains and protein three-dimensional structure. Nucleic Acids Res 41:D348–D352. doi:10.1093/nar/gks1243
Meier EL, Goley ED (2014) Form and function of the bacterial cytokinetic ring. Curr Opin Cell Biol 26:19–27. doi:10.1016/j.ceb.2013.08.006
Methé BA, Nelson KE, Deming JW et al (2005) The psychrophilic lifestyle as revealed by the genome sequence of Colwellia psychrerythraea 34H through genomic and proteomic analyses. PNAS 102:10913–10918. doi:10.1073/pnas.0504766102
Mykytczuk NCS, Wilhelm RC, Whyte LG (2012) Planococcus halocryophilus sp. nov., an extreme sub-zero species from high Arctic permafrost. Int J Syst Evol Microbiol 62:1937–1944. doi:10.1099/ijs.0.035782-0
Mykytczuk NCS, Foote SJ, Omelon CR et al (2013) Bacterial growth at −15 ℃; molecular insights from the permafrost bacterium Planococcus halocryophilus Or1. ISME J 7:1211–1226. doi:10.1038/ismej.2013.8
Olaya-Abril A, Jiménez-Munguía I, Gómez-Gascón L, Rodríguez-Ortega MJ (2014) Surfomics: shaving live organisms for a fast proteomic identification of surface proteins. J Proteomics 97:164–176. doi:10.1016/j.jprot.2013.03.035
Pakchung AAH, Soe CZ, Codd R (2008) Studies of iron-uptake mechanisms in two bacterial species of the shewanella genus adapted to middle-range (Shewanella putrefaciens) or antarctic (Shewanella gelidimarina) temperatures. Chem Biodivers 5:2113–2123. doi:10.1002/cbdv.200890192
Petersen TNT, Brunak SS, von Heijne GG, Nielsen HH (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8:785–786. doi:10.1038/nmeth.1701
Ramachandran V, Chandrakala B, Kumar VP et al (2006) Screen for inhibitors of the coupled transglycosylase-transpeptidase of peptidoglycan biosynthesis in Escherichia coli. Antimicrob Agents Chemother 50:1425–1432. doi:10.1128/AAC.50.4.1425-1432.2006
Riley M, Staley JT, Danchin A et al (2008) Genomics of an extreme psychrophile, Psychromonas ingrahamii. BMC Genom 9:210. doi:10.1186/1471-2164-9-210
Rivkina EM, Friedmann EI, McKay CP, Gilichinsky DA (2000) Metabolic activity of permafrost bacteria below the freezing point. Appl Environ Microbiol 66:3230–3233. doi:10.1128/AEM.66.8.3230-3233.2000
Rodríguez-Ortega MJ, Norais N, Bensi G et al (2006) Characterization and identification of vaccine candidate proteins through analysis of the group A Streptococcus surface proteome. Nat Biotechnol 24:191–197. doi:10.1038/nbt1179
Ronholm J, Schumann D, Sapers HM et al (2014) Mineralogical characterization of calcium carbonates precipitated by heterotrophic bacteria isolated from cryophilic polar regions. Geomicrobiology 12:542–556
Severin A, Nickbarg E, Wooters J, Quazi SA (2007) Proteomic analysis and identification of Streptococcus pyogenes surface-associated proteins. J Bacteriol 5:1514–1542
Solis N, Cordwell SJ (2011) Current methodologies for proteomics of bacterial surface-exposed and cell envelope proteins. Proteomics 11:3169–3189. doi:10.1002/pmic.201000808
Solis N, Larsen MR, Cordwell SJ (2010) Improved accuracy of cell surface shaving proteomics in Staphylococcus aureus using a false-positive control. Proteomics 10:2037–2049. doi:10.1002/pmic.200900564
Tjalsma H, Lambooy L, Hermans PW, Swinkels DW (2008) Shedding & shaving: disclosure of proteomic expressions on a bacterial face. Proteomics 8:1415–1428. doi:10.1002/pmic.200700550
Wilkins MRM, Gasteiger EE, Bairoch AA et al (1999) Protein identification and analysis tools in the ExPASy server. Methods Mol Biol 112:531–552. doi:10.1385/1-59259-584-7:531
Yu NYN, Wagner JRJ, Laird MRM et al (2010) PSORTb 3.0: improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 26:1608–1615. doi:10.1093/bioinformatics/btq249
Zhang CXY, Creskey MC, Cyr TD et al (2013) Proteomic identification of Listeria monocytogenes surface-associated proteins. Proteomics 13:3040–3045. doi:10.1002/pmic.201200449
Acknowledgments
Funding for this research was provided by the Natural Sciences and Engineering Research Council (NSERC), Canada Research Chair program (CRC), Canadian Foundation for Innovation (CFI), Polar Continental Shelf Program (PCSP), the Canadian Space Agency (CSA) Canadian Analogue Research Network (CARN) Program, and NSERC CREATE research/rotation support grants to JR and IRB.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Albers.
Rights and permissions
About this article
Cite this article
Ronholm, J., Raymond-Bouchard, I., Creskey, M. et al. Characterizing the surface-exposed proteome of Planococcus halocryophilus during cryophilic growth. Extremophiles 19, 619–629 (2015). https://doi.org/10.1007/s00792-015-0743-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-015-0743-4