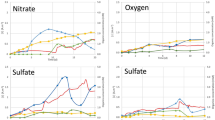
Abstract
Active hydrothermal chimneys host diverse microbial communities exhibiting various metabolisms including those involved in various biogeochemical cycles. To investigate microbe–mineral–fluid interactions in hydrothermal chimney and the driver of microbial diversity, a cultural approach using a gas-lift bioreactor was chosen. An enrichment culture was performed using crushed active chimney sample as inoculum and diluted hydrothermal fluid from the same vent as culture medium. Daily sampling provided time-series access to active microbial diversity and medium composition. Active archaeal and bacterial communities consisted mainly of sulfur, sulfate and iron reducers and hydrogen oxidizers with the detection of Thermococcus, Archaeoglobus, Geoglobus, Sulfurimonas and Thermotoga sequences. The simultaneous presence of active Geoglobus sp. and Archaeoglobus sp. argues against competition for available carbon sources and electron donors between sulfate and iron reducers at high temperature. This approach allowed the cultivation of microbial populations that were under-represented in the initial environmental sample. The microbial communities are heterogeneously distributed within the gas-lift bioreactor; it is unlikely that bulk mineralogy or fluid chemistry is the drivers of microbial community structure. Instead, we propose that micro-environmental niche characteristics, created by the interaction between the mineral grains and the fluid chemistry, are the main drivers of microbial diversity in natural systems.










Similar content being viewed by others
References
Alain K, Olagnon M, Desbuyères D, Page A, Barbier G, Juniper K, Querellou J, Cambon-Bonavita MA (2002) Phylogenetic characterization of the bacterial assemblage associated with the hydrothermal vent polychaete Paralvinella palmiformis. FEMS Microbiol Ecol 42:463–476
Alain K, Postec A, Grinsard E, Lesongeur F, Prieur D, Godfroy A (2010) Thermodesulfatator atlanticus sp. nov., a thermophilic, chemolithoautotrophic, sulfate-reducing bacterium isolated from a Mid-Atlantic Ridge hydrothermal vent. Int J Syst Evol Microbiol 60(1):33–38. doi:10.1099/ijs.0.009449-0
Bertoldo C, Antranikian G (2006) The order thermococcales. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds) The prokaryotes. Springer, New York, pp 69–81. doi:10.1007/0-387-30743-5_5
Blazewicz SJ, Barnard RL, Daly RA, Firestone MK (2013) Evaluating rRNA as an indicator of microbial activity in environmental communities: limitations and uses. ISME J 7(11):2061–2068. doi:10.1038/ismej.2013.102
Blöthe M, Roden EE (2009) Composition and activity of an autotrophic Fe(II)-oxidizing, nitrate-reducing enrichment culture. Appl Environ Microbiol 75(21):6937–6940. doi:10.1128/aem.01742-09
Boettger J, Lin H-T, Cowen JP, Hentscher M, Amend JP (2013) Energy yields from chemolithotrophic metabolisms in igneous basement of the Juan de Fuca ridge flank system. Chem Geol 337:11–19
Brettar I, Christen R, Höfle MG (2003) Idiomarina baltica sp. nov., a marine bacterium with a high optimum growth temperature isolated from surface water of the central Baltic Sea. Int J Syst Evol Microbiol 53(2):407–413
Burggraf S, Jannasch HW, Nicolaus B, Stetter KO (1990) Archaeoglobus profundus sp. nov., represents a new species within the sulfate-reducing archaebacteria. Syst Appl Microbiol 13:24–28
Byrne N, Lesongeur F, Bienvenu N, Geslin C, Alain K, Prieur D, Godfroy A (2009a) Effect of variation of environmental conditions on the microbial communities of deep-sea vent chimneys, cultured in a bioreactor. Extremophiles. 13 (Springer Japan)
Byrne N, Strous M, Crépeau V, Kartal B, Jean-Louis B, Schmid MC, Lesongeur F, Schouten S, Jaeschke A, Jetten MSM, Prieur D, Godfroy A (2009b) Presence and activity of anaerobic ammonium-oxidizing bacteria at deep-sea hydrothermal vents. ISME J 3:1–7
Callac N, Rommevaux-Jestin C, Rouxel O, Lesongeur F, Liorzou C, Bollinger C, Ferrant A, Godfroy A (2013) Microbial colonization of basaltic glasses in hydrothermal organic-rich sediments at Guaymas Basin. Front Microbiol 4. doi:10.3389/fmicb.2013.00250
Campbell BJ, Engel AS, Porter ML, Takai K (2006) The versatile ε-proteobacteria: key players in sulphidic habitats. Nat Rev Microbiol 4(6):458–468. http://www.nature.com/nrmicro/journal/v4/n6/suppinfo/nrmicro1414_S1.html
Campbell BJ, Polson SW, Allen LZ, Williamson SJ, Lee CK, Wommack KE, Cary SC (2013) Diffuse flow environments within basalt- and sediment-based hydrothermal vent ecosystems harbor specialized microbial communities. Front Microbiol 4:182. doi:10.3389/fmicb.2013.00182
Casamayor EO, Schafer H, Baneras L, Pedros-Alio C, Muyzer G (2000) Identification of and spatio-temporal differences between microbial assemblages from two neighboring sulfurous lakes: comparison by microscopy and denaturing gradient gel electrophoresis. Appl Environ Microbiol 66(2):499–508. doi:10.1128/aem.66.2.499-508.2000
Cline JD (1969) Spectrophotometric determination of hydrogen sulfide in naturals waters. Limnol Oceanogr 14:454–458
Colwell R (2013) EstimateS: statistical estimation of species richness and shared species from samples. Version 9 and earlier. User’s Guide and application. http://viceroy.eeb.uconn.edu/estimates/index.html
De la Lanza-Espino G, Soto LA (1999) Sedimentary geochemistry of hydrothermal vents in Guaymas Basin, Gulf of California, Mexico. Appl Geochem 14(4):499–510
Emerson D, Rentz JA, Lilburn TG, Davis RE, Aldrich H, Chan C, Moyer CL (2007) A novel lineage of proteobacteria involved in formation of marine Fe-oxidizing microbial mat communities. PLoS One 2(8):e667
Emerson D, Fleming EJ, McBeth JM (2010) Iron-oxidizing bacteria: an environmental and genomic perspective. Annu Rev Microbiol 64:561–583
Flores GE, Campbell JH, Kirshtein JD, Meneghin J, Podar M, Steinberg JI, Seewald JS, Tivey MK, Voytek MA, Yang ZK, Reysenbach A-L (2011) Microbial community structure of hydrothermal deposits from geochemically different vent fields along the Mid-Atlantic Ridge. Environ Microbiol 13(8):2158–2171. doi:10.1111/j.1462-2920.2011.02463.x
Flores G, Shakya M, Meneghin J, Yang Z, Seewald J, Geoff Wheat C, Podar M, Reysenbach AL (2012) Inter-field variability in the microbial communities of hydrothermal vent deposits from a back-arc basin. Geobiology 10(4):333–346
Godfroy A, Lesongeur F, Raguénès G, Quérellou J, Antoine E, Meunier J-R, Guezennec J, Barbier G (1997) Thermococcus hydrothermalis sp. nov., a new hyperthermophilic Archaeon isolated from deep-sea hydrothermal vent. Int J Syst Bacteriol 47:622–626
Godfroy A, Postec A, Raven N (2006) Growth of hyperthermophilic microorganisms for physiological and nutritional studies. In: Rainey FA, Oren A (eds) Methods in microbiology, extremophiles. Academic Press, Oxford
Good IJ (1953) The population frequencies of species and the estimation of population parameters. Biometrika 40(3–4):237–264
Hafenbradl D, Keller M, Dirmeier R, Rachel R, Rossnagel P, Burggraf S, Huber H, Stetter KO (1996) Ferroglobus placidus gen, nov., sp, nov., a novel hyperthermophilic archaeum that oxidizes Fe2+ at neutral pH under anoxic conditions. Arch Microbiol 166(5):308–314
Higashi Y, Sunamura M, Kitamura K, K-i Nakamura, Kurusu Y, J-i Ishibashi, Urabe T, Maruyama A (2004) Microbial diversity in hydrothermal surface to subsurface environments of Suiyo Seamount, Izu-Bonin Arc, using a catheter-type in situ growth chamber. FEMS Microbiol Ecol 47(3):327–336
Holden JF, Adams MWW (2003) Microbe–metal interactions in marine hydrothermal environments. Curr Opin Chem Biol 7(2):160–165
Houghton JL, Seyfried WE Jr (2010) An experimental and theoretical approach to determining linkages between geochemical variability and microbial biodiversity in seafloor hydrothermal chimneys. Geobiology 8(5):457–470. doi:10.1111/j.1472-4669.2010.00255.x
Houghton J, Seyfried W, Banta A, Reysenbach A (2007) Continuous enrichment culturing of thermophiles under sulfate and nitrate-reducing conditions and at deep-sea hydrostatic pressures. Extremophiles 11(2):371–382
Huber JA, Morrison HG, Huse SM, Neal PR, Sogin ML, Mark Welch DB (2009) Effect of PCR amplicon size on assessments of clone library microbial diversity and community structure. Environ Microbiol 11(5):1292–1302. doi:10.1111/j.1462-2920.2008.01857.x
Huo Y-Y, Xu X-W, Cao Y, Wang C-S, Zhu X-F, Oren A, Wu M (2009) Marinobacterium nitratireducens sp. nov. and Marinobacterium sediminicola sp. nov., isolated from marine sediment. Int J Syst Evol Microbiol 59(5):1173–1178. doi:10.1099/ijs.0.005751-0
Inagaki F, Motomura Y, Doi K, Taguchi S, Izawa E, Lowe DR, Ogata S (2001) Silicified microbial community at Steep Cone Hot Spring, Yellowstone National Park. Microbes Environ 16(2):125–130
Jeanthon C, L’Haridon S, Cueff V, Banta A, Reysenbach AL, Prieur D (2002) Thermodesulfobacterium hydrogeniphilum sp. nov., a thermophilic, chemolithoautotrophic, sulfate-reducing bacterium isolated from a deep-sea hydrothermal vent at Guaymas Basin, and emendation of the genus Thermodesulfobacterium. Int J Syst Evol Microbiol 52(3):765–772
Jost G, Martens-Habbena W, Pollehne F, Schnetger B, Labrenz M (2010) Anaerobic sulfur oxidation in the absence of nitrate dominates microbial chemoautotrophy beneath the pelagic chemocline of the eastern Gotland Basin, Baltic Sea. FEMS Microbiol Ecol 71(2):226–236. doi:10.1111/j.1574-6941.2009.00798.x
Karl DM (1995) Ecology of free-living, hydrothermal vent microbial communities. In: Karl DM (ed) The microbiology of deep-sea hydrothermal vents. CRC Press, Boca Raton, pp 35–124
Kashefi K, Tor JM, Holmes DE, Gaw Van Praagh CV, Reysenbach AL, Lovley DR (2002) Geoglobus ahangari gen. nov., sp. nov., a novel hyperthermophilic archaeon capable of oxidizing organic acids and growing autotrophically on hydrogen with Fe(III) serving as the sole electron acceptor. Int J Syst Evol Microbiol 52(3):719–728
Kashefi K, Holmes DE, Baross JA, Lovley DR (2003) Thermophily in the Geobacteraceae: geothermobacter ehrlichii gen. nov., sp. nov., a novel thermophilic member of the Geobacteraceae from the “Bag City” hydrothermal vent. Appl Environ Microbiol 69(5):2985–2993
Kato S, Kobayashi C, Kakegawa T, Yamagishi A (2009) Microbial communities in iron–silica-rich microbial mats at deep-sea hydrothermal fields of the Southern Mariana Trough. Environ Microbiol 11(8):2094–2111. doi:10.1111/j.1462-2920.2009.01930.x
Kato S, Takano Y, Kakegawa T, Oba H, Inoue K, Kobayashi C, Utsumi M, Marumo K, Kobayashi K, Ito Y, J-i Ishibashi, Yamagishi A (2010) Biogeography and biodiversity in sulfide structures of active and inactive vents at deep-sea hydrothermal fields of the Southern Mariana Trough. Appl Environ Microbiol 76(9):2968–2979. doi:10.1128/aem.00478-10
Kato S, Nakamura K, Toki T, Ishibashi J-I, Tsunogai U, Hirota A, Ohkuma M, Yamagishi A (2012) Iron-based microbial ecosystem on and below the seafloor: a case study of hydrothermal fields of the Southern Mariana Trough. Front Microbiol 3. doi:10.3389/fmicb.2012.00089
Kerr RC (1997) Heat transfer and hydrothermal fluid flow at flanges on large seafloor sulphide structures. Earth Planet Sci Lett 152(1–4):93–99
Kim H, Choo Y-J, Song J, Lee J-S, Lee KC, Cho J-C (2007) Marinobacterium litorale sp. nov. in the order Oceanospirillales. Int J Syst Evol Microbiol 57(7):1659–1662. doi:10.1099/ijs.0.64892-0
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16(2):111–120. doi:10.1007/bf01731581
Kolganova TV, Kuznetsov BB, Tourova TP (2002) Designing and testing oligonucleotide primers for amplification and sequencing of archaeal 16S rRNA genes. Microbiology 71(2):243–246. doi:10.1023/a:1015122926687
Kormas KA, Tivey MK, Von Damm K, Teske A (2006) Bacterial and archaeal phylotypes associated with distinct mineralogical layers of a white smoker spire from a deep-sea hydrothermal vent site (9°N, East Pacific Rise). Environ Microbiol 8(5):909–920. doi:10.1111/j.1462-2920.2005.00978.x
Lai Q, Wang L, Liu Y, Yuan J, Sun F, Shao Z (2011) Parvibaculum indicum sp. nov., isolated from deep-sea water. Int J Syst Evol Microbiol 61(2):271–274
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, Chichester, pp 115–175
Lane DJ, Pace B, Olsen GJ, Stahl DA, Sogin ML, Pace NR (1985a) Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc Natl Acad Sci 82(20):6955–6959
Lane DJ, Pace B, Olsen GJ, Sthal DA, Sogin ML, Pace NRO (1985b) Rapid determination of 16S ribosomal RNA sequences for phylogenic analysis. Proc Natl Acad Sci USA 82:6955–6959
Lazar CS, Parkes RJ, Cragg BA, L’Haridon S, Toffin L (2011) Methanogenic diversity and activity in hypersaline sediments of the centre of the Napoli mud volcano, Eastern Mediterranean Sea. Environ Microbiol 13(8):2078–2091. doi:10.1111/j.1462-2920.2011.02425.x
Lee ZM-P, Bussema C, Schmidt TM (2009) rrnDB: documenting the number of rRNA and tRNA genes in bacteria and archaea. Nucleic Acids Res 37(suppl 1):D489–D493
Lozupone C, Hamady M, Knight R (2006) UniFrac—an online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinform 7(1):371
Maestrojuan GM, Boone DR, Xun L, Mah RA, Zhang L (1990) Transfer of Methanogenium bourgense, Methanogenium marisnigri, Methanogenium olentangyi, and Methanogenium thermophilicum to the genus Methanoculleus gen. nov., emendation of Methanoculleus marisnigri and Methanogenium, and description of new strains of Methanoculleus bourgense and Methanoculleus marisnigri. Int J Syst Bacteriol 40(2):117–122. doi:10.1099/00207713-40-2-117
Martens CS (1990) Generation of short chain acid anions in hydrothermally altered sediments of the Guaymas Basin, Gulf of California. Appl Geochem 5(1–2):71–76
McCollom TM (2007) Geochemical constraints on sources of metabolic energy for chemolithoautotrophy in ultramafic-hosted deep-sea hydrothermal systems. Astrobiology 7(6):933–950
McCollom TM, Shock EL (1997) Geochemical constraints on chemolithoautotrophic metabolism by microorganisms in seafloor hydrothermal systems. Geochim Cosmochim Acta 61(20):4375–4391
McHatton SC, Barry JP, Jannasch HW, Nelson DC (1996) High nitrate concentrations in vacuolate, autotrophic marine Beggiatoa spp. Appl Environ Microbiol 62(3):954–958
Miroshnichenko ML, Slobodkin AI, Kostrikina NA, L’Haridon S, Nercessian O, Spring S, Stackebrandt E, Bonch-Osmolovskaya EA, Jeanthon C (2003) Deferribacter abyssi sp. nov., an anaerobic thermophile from deep-sea hydrothermal vents of the Mid-Atlantic Ridge. Int J Syst Evol Microbiol 53(5):1637–1641
Moussard H, L’Haridon S, Tindall BJ, Banta A, Schumann P, Stackebrandt E, Reysenbach A-L, Jeanthon C (2004) Thermodesulfatator indicus gen. nov., sp. nov., a novel thermophilic chemolithoautotrophic sulfate-reducing bacterium isolated from the Central Indian Ridge. Int J Syst Evol Microbiol 54(1):227–233
Muyzer G, De Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
Nakagawa S, Takai K, Inagaki F, Hirayama H, Nunoura T, Horikoshi K, Sako Y (2005) Distribution, phylogenetic diversity and physiological characteristics of epsilon-Proteobacteria in a deep-sea hydrothermal field. Environ Microbiol 7(10):1619–1632
Nakamura K, Takai K (2014) Theoretical constraints of physical and chemical properties of hydrothermal fluids on variations in chemolithotrophic microbial communities in seafloor hydrothermal systems. Prog Earth Planet Sci 1(1):1–24
Olins H, Rogers D, Frank K, Vidoudez C, Girguis P (2013) Assessing the influence of physical, geochemical and biological factors on anaerobic microbial primary productivity within hydrothermal vent chimneys. Geobiology 11(3):279–293
Page A, Tivey MK, Stakes DS, Reysenbach A-L (2008) Temporal and spatial archaeal colonization of hydrothermal vent deposits. Environ Microbiol 10(4):874–884. doi:10.1111/j.1462-2920.2007.01505.x
Peter JM, Scott SD (1988) Mineralogy, composition, and fluid inclusion microthermometry of sea-floor hydrothermal deposits in the southern trough of Guaymas Basin, Gulf of California. Can Mineral 26(3):567–587
Postec A, Urios L, Lesongeur F, Ollivier B, Quérellou J, Godfroy A (2005) Continuous enrichment culture and molecular monitoring to investigate the microbial diversity of thermophiles inhabiting the deep-sea hydrothermal ecosystems. Curr Microbiol 50:138–144
Postec A, Lesongeur F, Pignet P, Ollivier B, Quérellou J, Godfroy A (2007) Continuous enrichment cultures: insights into prokaryotic diversity and metabolic interactions in deep-sea vent chimneys. Extremophiles 11:747–757
Reysenbach A-L, Cady SL (2001) Microbiology of ancient and modern hydrothermal systems. Trends Microbiol 9(2):79–86
Reysenbach AL, Longnecker K, Kirshtein J (2000) Novel bacterial and archaeal lineages from an in situ growth chamber deployed at a Mid-Atlantic Ridge hydrothermal vent. Appl Environ Microbiol 66(9):3798–3806
Rickard D (1995) Kinetics of FeS precipitation: part 1. Competing reaction mechanisms. Geochim Cosmochim Acta 59(21):4367–4379
Rosario-Passapera R, Keddis R, Wong R, Lutz RA, Starovoytov V, Vetriani C (2012) Parvibaculum hydrocarboniclasticum sp. nov., a mesophilic, alkane-oxidizing alphaproteobacterium isolated from a deep-sea hydrothermal vent on the East Pacific Rise. Int J Syst Evol Microbiol. doi:10.1099/ijs.0.039594-0
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4(4):406–425
Sarradin P-M, Waeles M, Bernagout S, Le Gall C, Sarrazin J, Riso R (2009) Speciation of dissolved copper within an active hydrothermal edifice on the Lucky Strike vent field (MAR, 37 N). Sci Total Environ 407(2):869–878
Sayeh R, Birrien J, Alain K, Barbier G, Hamdi M, Prieur D (2010) Microbial diversity in Tunisian geothermal springs as detected by molecular and culture-based approaches. Extremophiles 14(6):501–514. doi:10.1007/s00792-010-0327-2
Schleheck D, Weiss M, Pitluck S, Bruce D, Land ML, Han S, Saunders E, Tapia R, Detter C, Brettin T (2011) Complete genome sequence of Parvibaculum lavamentivorans type strain (DS-1T). Stand Genomic Sci 5(3):298
Simoneit BRT, Lonsdale PF (1982) Hydrothermal petroleum in mineralized mounds at the seabed of Guaymas Basin. Nature 295(5846):198–202
Slobodkin A, Campbell B, Cary SC, Bonch-Osmolovkaya EA, Jeanthon C (2001) Evidence for the presence of thermophilic Fe(III)-reducing microorganisms in deep-sea hydrothermal vents at 13°N (East Pacific Rise). FEMS Microbiol Ecol 36:235–243
Slobodkina GB, Kolganova TV, Chernyh NA, Querellou J, Bonch-Osmolovskaya EA, Slobodkin AI (2009a) Deferribacter autotrophicus sp. nov., an iron(III)-reducing bacterium from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol 59(6):1508–1512
Slobodkina GB, Kolganova TV, Querellou J, Bonch-Osmolovskaya EA, Slobodkin AI (2009b) Geoglobus acetivorans sp. nov., an iron(III)-reducing archaeon from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol 59(11):2880–2883
Sorokin DY, Tourova TP, Muyzer G (2005) Citreicella thiooxidans gen. nov., sp. nov., a novel lithoheterotrophic sulfur-oxidizing bacterium from the Black Sea. Syst Appl Microbiol 28(8):679–687
Straub KL, Benz M, Schink B, Widdel F (1996) Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Appl Environ Microbiol 62(4):1458–1460
Suzuki MT, Taylor LT, DeLong EF (2000) Quantitative analysis of small-subunit rRNA genes in mixed microbial populations via 5′-nuclease assays. Appl Environ Microbiol 66(11):4605–4614. doi:10.1128/aem.66.11.4605-4614.2000
Takai K, Komatsu T, Inagaki F, Horikoshi K (2001) Distribution of archaea in a black smoker chimney structure. Appl Environ Microbiol 67(8):3618–3629. doi:10.1128/aem.67.8.3618-3629.2001
Takai K, Nunoura T, Ishibashi J-I, Lupton J, Suzuki R, Hamasaki H, Ueno Y, Kawagucci S, Gamo T, Suzuki Y, Hirayama H, Horikoshi K (2008) Variability in the microbial communities and hydrothermal fluid chemistry at the newly discovered Mariner hydrothermal field, southern Lau Basin. J Geophys Res 113. doi:10.1029/2007JG000521
Teske A, Sorensen KB (2007) Uncultured archaea in deep marine subsurface sediments: have we caught them all? ISME J 2(1):3–18. http://www.nature.com/ismej/journal/v2/n1/suppinfo/ismej200790s1.html
Teske A, Hinrichs K-U, Edgcomb V, de Vera Gomez A, Kysela D, Sylva SP, Sogin ML, Jannasch HW (2002) Microbial diversity of hydrothermal sediments in the Guaymas Basin: evidence for anaerobic methanotrophic communities. Appl Environ Microbiol 68(4):1994–2007
Teske A, Edgcomb V, Rivers A, Thompson J, de Vera Gomez A, Molyneaux S, Wirsen C (2009) A molecular and physiological survey of a diverse collection of hydrothermal vent Thermococcus and Pyrococcus isolates. Extremophiles 13(6):905–915
Tivey MK, Delaney JR (1986) Growth of large sulfide structures on the endeavour segment of the Juan de Fuca ridge. Earth Planet Sci Lett 77(3–4):303–317
Tivey MK, Stakes DS, Cook TL, Hannington MD, Petersen S (1999) A model for growth of steep-sided vent structures on the endeavour segment of the Juan de Fuca Ridge: results of a petrologic and geochemical study. J Geophys Res 104(B10):22859–22883. doi:10.1029/1999jb900107
Toner BM, Lesniewski RA, Marlow JJ, Briscoe LJ, Santelli CM, Bach W, Orcutt BN, Edwards KJ (2013) Mineralogy drives bacterial biogeography of hydrothermally inactive seafloor sulfide deposits. Geomicrobiol J 30(4):313–326
Vargas M, Kashefi K, BluntHarris EL, Lovley DR (1998) Microbiological evidence for Fe(III) reduction on early Earth. Nature 395(6697):65–67
Vazquez GF, Zhang J, zhong, Millero FJ (1989) Effect of metals on the rate of the oxidation of H2S in seawater. Geophys Res Lett 16(12):1363–1366. doi:10.1029/GL016i012p01363
Von Damm KL, Edmond JM, Measures CI, Grant B (1985) Chemistry of submarine hydrothermal solutions at Guaymas Basin, Gulf of California. Geochim Cosmochim Acta 49:2221–2237
Wagner M, Roger AJ, Flax JL, Brusseau GA, Stahl DA (1998) Phylogeny of Dissimilatory sulfite reductases supports an early origin of sulfate respiration. J Bacteriol 180(11):2975–2982
Wang F, Zhou H, Meng J, Peng X, Jiang L, Sun P, Zhang C, Van Nostrand JD, Deng Y, He Z, Wu L, Zhou J, Xiao X (2009) GeoChip-based analysis of metabolic diversity of microbial communities at the Juan de Fuca Ridge hydrothermal vent. Proc Natl Acad Sci 106(12):4840–4845. doi:10.1073/pnas.0810418106
Welhan JA (1988) Origins of methane in hydrothermal systems. Chem Geol 71(1–3):183–198
Wintzingerode F, Göbel UB, Stackebrandt E (1997) Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol Rev 21(3):213–229
Woods AW, Delaney JR (1992) The heat and fluid transfer associated with the flanges on hydrothermal venting structures. Earth Planet Sci Lett 112(1–4):117–129
Yamamoto M, Takai K (2011) Sulfur metabolisms in epsilon- and gamma-Proteobacteria in deep-sea hydrothermal fields. Front Microbiol 2. doi:10.3389/fmicb.2011.00192
Yu Y, Lee C, Kim J, Hwang S (2005) Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol Bioeng 89(6):670–679. doi:10.1002/bit.20347
Acknowledgments
We want to thank all the shipboard cruise party for their work and support during the BIG cruise: officers, crew and technicians of the R/V L’Atalante, the DSV Nautile team and the on-board scientific team. This cruise was funded by IFREMER (France) and has benefited from a work permit in Mexican waters (DAPA/2/281009/3803, October 28th, 2009). We also acknowledge the anonymous reviewers for their constructive suggestions and comments. We are grateful to Carole Decker and Jean-Claude Caprais for their assistance with hydrogen sulfide measurement. Yoan Germain is thanked for clean laboratory assistance and Karine Estève for her help during the cruise. We also thank Pierre-Marie Sarradin for provide us the PEPITO’s temperature data. This work was supported by Ifremer, the GIS Europôle Mer, UEB, CNRS, and has benefited from state aid managed by the Agence Nationale de la Recherche under the program “Investments for the Future” with the reference ANR-10-LabX-19-01.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Atomi.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Callac, N., Rouxel, O., Lesongeur, F. et al. Biogeochemical insights into microbe–mineral–fluid interactions in hydrothermal chimneys using enrichment culture. Extremophiles 19, 597–617 (2015). https://doi.org/10.1007/s00792-015-0742-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-015-0742-5