Abstract
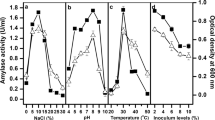
A halophilic archaeon, Halorubrum sp. strain Ha25, produced extracellular halophilic organic solvent-tolerant amylopullulanase. The maximum enzyme production was at high salt concentration, 3–4 M NaCl. Optimum pH and temperature for enzyme production were 7.0 and 40 °C, respectively. Molecular mass of purified enzyme was estimated to be about 140 kDa by SDS–PAGE. This enzyme was active on pullulan and starch as substrates. The apparent K m for the enzyme activity on pullulan was 4 mg/ml and for soluble starch was 1.8 mg/ml. Optimum temperature for amylolytic and pullulytic activities was 50 °C. Optimum pH for amylolytic activity was 7 and for pullulytic activity was 7.5. This enzyme was active over a wide range of concentrations (0–4.5 M) of NaCl. The effect of organic solvents on the enzyme activities showed that this enzyme was more stable in the presence of non-polar organic solvents than polar solvents. This study is the first report on amylopullulanase production in halophilic bacteria and archaea.




Similar content being viewed by others
References
Adams MWW, Kelly RM (1995) Enzymes from microorganisms in extreme environments. Chem Eng News 73:32–42
Al-ZaZaee MM, Gurumurthy DM, Rajeshwara AN (2011) Identification, characterization of novel halophilic Bacillus Cereus Ms6: a source for extra cellular A-amylase. Adv Environ Biol 5:992–999
Boone DR, Castenhoiz RW, Garrity GM (2001( Bergey’s manual of systematic bacteriology: The Archaea and the deeply branching and phototrophic bacteria. Springer, New York
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chung YC, Kobayashi T, Kanai H, Akiba T, Kudo T (1995) Purification and properties of extracellular amylase from the hyperthermophilic archaeon Thermococcus profundus DT5432. Appl Environ Microbiol 61:1502–1506
Coronado MJ, Vargas C, Hofemeister J, Ventosa A, Nieto J (2000) Production and biochemical characterization of an α-amylase from the moderate halophile Halomonas meridiana. FEMS Microbiol Lett 183:67–71
Danson MJ, Hough DW (1997) The structural basis of protein halophilicity. Comp Biochem Physiol 117:307–312
Diger A, Antranikian G (1995) Isolation and characterization of a heat-stable pullulanase from the hyperthermophilic archaeon Pyrococcus woesei after cloning and expression of its gene in Escherichia coli. Appl Environ Microbiol 61:567–575
Domań-Pytka M, Bardowski J (2004) Pullulan degrading enzymes of bacterial origin. Crit Rev Microbiol 30:107–121
Dong G, Vieille C, Zeikus G (1997) Cloning, sequencing, and expression of the gene encoding amylopullulanase from Pyrococcus furiosus and biochemical characterization of the recombinant enzyme. Appl Environ Microbiol 63:3577–3584
Doukyua N, Ogino H (2010) Organic solvent-tolerant enzymes. Biochem Eng J 48:270–282
Duffner F, Bertoldo C, Andersen JT, Wagner K, Antranikian G (2000) A new thermoactive pullulanase from Desulfurococcus mucosus: cloning, sequencing, purification, and characterization of the recombinant enzyme after expression in Bacillus subtilis. J Bacteriol 182:6331–6338
Dussault H (1955) An improved technique for staining red halophilic bacteria. J Bacteriol 70:484
Dyall-Smith M (2009) The Halohandbook: protocols for halobacterial genetics. Haloarchaeal Genetics Laboratory, Melbourne
Forbes B, Sahm D, Weissfeld A (2007) Bailey and Scott’s diagnostic microbiology. 12th edn. Mosby Inc., St. Louis, pp 80–89
Fukushima T, Echigo A, Inoue A, Usami R (2005) Organic solvent tolerance of halophilic α-amylase from a haloarchaeon, Haloarcula sp. strain S-1. Extremophiles 9:85–89
Ganghofner D, Kellermann J, Staudenbauer W, Bronnenmeier K (1998) Purification and properties of amylopullulanase, a glucoamylase and an alpha glucosidase in the amylolytic enzyme system of Thermoanaerobacterium thermosaccharolyticum. Biosci Biotechnol Biochem 62:302–308
Ghollasi M, Khajeh K, Mollania N, Zareian S, Naderi-Manesh H (2008) An investigation on acarbose inhibition and the number of active sites in an amylopullulanase (L14-APU) from an Iranian Bacillus sp. Biologia 63(6):1051–1056
Gomes I, Gomes J, Steiner W (2003) Highly thermostable amylase and pullulanase of the extreme thermophilic eubacterium Rhodothermus marinus: production and partial characterization. Bioresour Technol 90:207–214
Grant WD, Kamekura M, McGenity TJ, Ventosa A (2001) Bergey’s manual of systematic bacteriology: the archaea and deeply branching and phototrophic bacteria, vol 1, 2nd edn. Springer, New York
Gutiérrez C, González C (1972) Method for simultaneous detection of proteinase and esterase activities in extremely halophilic bacteria. Appl Microbiol 24:516–517
Hatada Y, Igarashi K, Ozaki K, Ara K, Hitomi J, Kobayashi T, Kawai S, Watabe T, Ito S (1996) Amino acid sequence and molecular structure of an alkaline amylopullulanase from Bacillus that hydrolyzes α-1,4 and α-1,6 linkages in polysaccharides at different active sites. J Biol Chem 271:24075–24083
Hutcheon GW, Bolhuis A (2005) Characterisation of a highly stable a-amylase from the halophilic archaeon Haloarcula hispanica. Extremophiles 9:487–495
Jiao Y, Ming-Sheng L, Xu J, Fang Y (2011) A GH57 family amylopullulanase from deep-sea Thermococcus siculi: expression of the gene and characterization of the recombinant enzyme. Curr Microbiol 62:222–228
Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi H, Won S, Chun J (2012) Introducing EzTaxon-e: a prokaryotic 16S rRNA Gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol 62:716–721
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Makhdoumi Kakhki A, Amoozegar MA, Mahmodi Khaledi E (2011) Diversity of hydrolytic enzymes in haloarchaeal strains isolated from salt lake. Int J Environ Sci Tech 8:705–714
Mancinelli RL, Landheim R, Sanchez-Porro C, Dornmayr-Pfaffenhuemer M, Gruber C, Legat A, Ventosa A, Radax C, Ihara K, White M, Stan-Lotter H (2009) Halorubrum chaoviator sp. nov., a haloarchaeon isolated from sea salt in Baja California, Mexico, Western Australia and Naxos, Greece. Int J Syst Evol Microbiol 59:1908–1913
Margesin R, Schinner F (2001) Potential of halotolerant and halophilic microorganisms for biotechnology. Extremophiles 5:73–83
Mathupala S, Lowe S, Gregory Zeikus J (1993) Sequencing of the amylopullulanase (apu) gene of Therrnoanaerobacter ethanolicus 39E, and identification of the active site by site-directed mutagenesis. J Biol Chem 268:16332–16334
Melasneimi H (1987) Effect of carbon source on production of thermostable a-amylase, pullulanase and a-glucosidase by Clostridium thermohydrosulfuricum. J Gen Microbiol 133:883–890
Mevarech M, Frolow F, Gloss LM (2000) Halophilic enzymes: proteins with a grain of salt. Biophys Chem 86:155–164
Miller G (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem 31:426–428
Mrudula S, Reddy G, Seenayya G (2011) Purification and characterization of highly thermostable amylopullulanase from a thermophilic, anaerobic bacterium Clostridium thermosulfurogenes SVM17. MJM 7(2):97–106
Nair SU, Singhal RS, Kamat MY (2006) Enhanced production of thermostable pullulanase type 1 using Bacillus cereus FDTA 13 and its mutant. Food Technol Biotechnol 44(2):275–282
Nair SU, Singhal RS, Kamat MY (2007) Induction of pullulanase production in Bacillus cereus FDTA-13. Bioresour Technol 98:856–859
Niehaus F, Bertoldo C, Ka¨hler M, Antranikian G (1999) Extremophiles as a source of novel enzymes for industrial application. Appl Microbiol Biotechnol 51:711–729
Oren A, Ventosa A, Grant W (1997) Proposed minimal standards for description of new taxa in the order Halobacteriales. Int J Syst Evol Microbiol 47:233–238
Perez-Pomares F, Ferrer J, Pire C, Marhuenda-Egea FC, Bonete MJ (2003) α-Amylase activity from the halophilic archaeon Haloferax mediterranei. Extremophiles 7:299–306
Prakash B, Madhukumar MS, Muralikrishna G, Sreeramulu K (2009) Production, purification, and characterization of two extremely halotolerant, thermostable, and alkali-stable a-amylases from Chromohalobacter sp. TVSP 101. Process Biochem 44:210–215
Saha B, Lamed R, Lee C, Mathupala S (1990) Characterization of an endo-acting amylopullulanase from Thermoanaerobacter Strain B6A. Appl Environ Microbiol 56:881–886
Sata H, Umeda M, Kim C-H, Taniguchi H, Maruyama Y (1989) Amylase-pullulanase enzyme produced by B. circulans F-2. Biochimica et Biophysica Acta (BBA) General Subjects 991:388–394
Shafiei M, Ziaee A, Amoozegar MA (2011) Purification and characterization of an organic-solvent-tolerant halophilic a-amylase from the moderately halophilic Nesterenkonia sp. strain F. J Ind Microbiol Biotechnol 38:275–281
Sivaramakrishnan S, Gangadharan D, Nampoothiri KM, Soccol CR, Pandey A (2006) α-Amylases from microbial sources-an overview on recent developments. Food Technol Biotechnol 44:173–184
Xin L, Yu YY (2012) Characterization of an organic solvent-tolerant α-amylase from a halophilic isolate, Thalassobacillus sp. LY18. Folia Microbiol 57:447–453
Zareian S, Khajeh K, Ranjbar B, Dabirmanesh B, Ghollasi M, Mollania N (2010) Purification and characterization of a novel amylopullulanase that converts pullulan to glucose, maltose, and maltotriose and starch to glucose and maltose. Enzyme Microbial Technol 46:57–63
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Atomi.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Siroosi, M., Amoozegar, M.A., Khajeh, K. et al. Purification and characterization of a novel extracellular halophilic and organic solvent-tolerant amylopullulanase from the haloarchaeon, Halorubrum sp. strain Ha25. Extremophiles 18, 25–33 (2014). https://doi.org/10.1007/s00792-013-0589-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-013-0589-6