Abstract
Understanding the biological mechanisms behind multimorbidity patterns in adolescence is important as they may act as intermediary risk factor for long-term health. We aimed to explore relationship between prenatal exposures and adolescent’s psycho-cardiometabolic intermediary traits mediated through epigenetic biomarkers, using structural equation modeling (SEM). We used data from mother–child dyads from pregnancy and adolescents at 16–17 years from two prospective cohorts: Northern Finland Birth Cohort 1986 (NFBC1986) and Raine Study from Australia. Factor analysis was applied to generate two different latent factor structures: (a) prenatal exposures and (b) adolescence psycho-cardiometabolic intermediary traits. Furthermore, three types of epigenetic biomarkers were included: (1) DNA methylation score for maternal smoking during pregnancy (DNAmMSS), (2) DNAm age estimate PhenoAge and (3) DNAm estimate for telomere length (DNAmTL). Similar factor structure was observed between both cohorts yielding three prenatal factors, namely BMI (Body Mass Index), SOP (Socio-Obstetric-Profile), and Lifestyle, and four adolescent factors: Anthropometric, Insulin-Triglycerides, Blood Pressure, and Mental health. In the SEM pathways, stronger direct effects of F1prenatal-BMI (NFBC1986 = β: 0.27; Raine = β: 0.39) and F2prenatal-SOP (β: −0.11) factors were observed on adolescent psycho-cardiometabolic multimorbidity. We observed an indirect effect of prenatal latent factors through epigenetic markers on a psycho-cardiometabolic multimorbidity factor in Raine study (P < 0.05). The present study exemplifies an evidence-based approach in two different birth cohorts to demonstrate similar composite structure of prenatal exposures and psycho-cardiometabolic traits (despite cultural, social, and genetic differences) and a common plausible pathway between them through underlying epigenetic markers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
People with poor physical health often experience concurrent mental health concerns [1]. Much of the attention in this area is focused on adults, when cardiometabolic health outcomes become more apparent. However, upcoming evidence suggests that cardiometabolic risk can be identified at younger age, thus affording the opportunity to intervene to prevent comorbidities [2]. While gestation is an important developmental phase, adolescence is a critical transition period, characterized by rapid and ordinated developmental stages, only second to early childhood in its rate and breadth of effect upon mental and biological health concerns [3, 4].
The risk is posited to spiral from one generation to another, arising from adverse biological and environmental factors during gestation. Various in utero adversities together have immediate consequences on birth outcomes and are also known to influence the future burden of psycho-cardio-metabolic disorders [5,6,7]. Along these lines, in our previous study, we identified inverse association between maternal prenatal biopsychosocial score and birth weight [8], which is a known risk factor for numerous diseases across the lifespan [9]. In the study, four biopsychosocial latent factors, namely Factor1-BMI, Factor2-DBP (Diastolic Blood Pressure), Factor3-Socioeconomic-Obstetric-Profile (SOP), and Factor4-Parental-Lifestyle were derived using multiple in utero measures and the factor scores from each latent factor were combined into a cumulative score.
The link between heritability and environmental determinants of psycho-cardiometabolic traits, such as glucose intolerance, lipids, anxiety, and depression, is commonly explained through the epigenetic variability related to influences of the early life adversities as one of the plausible mechanisms [10, 11]. One such environmental determinant is maternal smoking during pregnancy, a well-established early life exposure strongly associated with the epigenetic variability in offspring until later life [12,13,14]. These differential epigenetic changes also mediate the association of prenatal maternal smoking on lower birth weight [15] and were subsequently shown to have long-term association with cardiometabolic intermediary traits [13] and psycho-cardiometabolic disorders [16]. Moving forward our research group has generated and evaluated a novel DNA methylation (DNAm) risk score in adolescents aiming to predict fetal exposures to maternal smoking during pregnancy (DNAmMSS: DNAm maternal smoking score during pregnancy) [17] and evaluate their subsequent risk upon future cardiometabolic diseases. This offers insight on the underlying paradigm of ‘biological embedding’ to diseases and may enable identifying individuals at risk of unknown exposures.
Epigenetic signatures using DNAm levels at age-related DNAm sites have been developed to capture aspects of biological aging. These DNAm age estimates vary within individuals of the same chronological age based on the incidence of age-related chronic diseases [18]. Several DNAm age estimates have been developed and among them PhenoAge is a score using 513 CpG sites to estimate “phenotypic age” derived from a set of clinical biomarkers of aging and it correlates well with chronological age [19]. Telomere Length (TL) is another important marker of biological age, as average TL decreases with age. Lu et al. developed a DNA methylation estimator of TL (DNAmTL: DNAm estimate for TL) based on 140 CpGs which was related to age-related pathologies [20]. Both PhenoAge and DNAmTL are observed to perform better than other DNAm scores in detecting the association with age, sex, behavioral factors, and numerous clinical intermediary traits as well as intrauterine exposures [19, 20].
Based on these previous studies [8, 17], this study aims to further expand on modeling latent constructs of prenatal exposures and adolescent’s psycho-cardiometabolic intermediary traits to test the pathways between them using different intermediary epigenetic signatures. Our specific objectives are to: (1) develop prenatal exposure and adolescent psycho-cardiometabolic comorbidities latent construct; (2) identify shared pathways from early life leading to adolescent psycho-cardiometabolic multimorbidity using epigenetic biomarkers: DNAmMSS, PhenoAge, and DNAm TL; and (3) cross-validate the findings between two birth cohorts: Northern Finland Birth Cohort 1986 (NFBC1986) and the Raine Study.
Methods
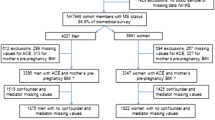
The data were derived from two prospective cohorts: NFBC1986 and the Raine Study. The NFBC1986 is a pregnancy-birth cohort consisting of 99% (N = 9215) of all children born in the recruitment zone (provinces of Oulu and Lapland) in the Northern Finland between 1 July 1985 and 30 June 1986 [21]. Offspring have been followed up until the age of 16 years, and the data were collected through a clinical examination (n = 5654) and postal questionnaires (n = 7344).
The Raine Study is a longitudinal Western Australia Pregnancy Cohort established in 1989 [22, 23]. From 1989 to 1991, pregnant women (N = 2900, Gen1) were recruited at King Edward Memorial Hospital and surrounding private hospitals. Of which 2,868 live births (Gen2) are followed up from 18 weeks’ gestation into young adulthood at multiple time points in order to investigate the early origins of adult disease through anthropometric, clinical, biochemical, and questionnaire data.
The present study included participants from singleton births and with complete data at each time point. Availability of DNA methylation data was the main reason for attrition in the sample size.
Measures
Prenatal exposures
In the NFBC1986, antenatal data were collected at 12th, 20th, and 36th week of pregnancy and in the Raine Study from antenatal visits at 18th and 34th week of gestation. In both cohorts, maternal pre-and-end pregnancy body mass index (BMI-kg/m2) was calculated through height and weight measured at the time of enrollment and at 36th/34th week of gestation. Maternal age, marital status, parity, alcohol use, and maternal and paternal smoking were self-reported through questionnaire. The measures were coded into dichotomous variables as: ‘married and unmarried (including single and widow)’; ‘nulliparous and multiparous’; ‘any maternal smoking during pregnancy and no smoking during pregnancy’; father smoking: ‘yes or no’; ‘any maternal alcohol use during pregnancy and no alcohol use’.
Epigenetic mediators
In the NFBC1986, DNA was extracted from all 5,654 whole blood samples available at the 16-year follow-up. Of these, DNA methylation for 546 randomly selected participants with complete follow-up data available was measured on Illumina Infinium HumanMethlation450K array (Illumina, San Diego, USA) at the Department of Genomics Imperial College London (London, UK). After quality control and based on variable availability, 490 samples were used in the analyses [16]. In Raine Study, at the 17-year follow-up, DNA methylation was measured in peripheral whole blood sample of 996 European ancestry participants using the same Illumina Infinium HumanMethylation450K BeadChip [24]. We included three epigenetic scores in this study: DNAmMSS [17], DNAm age estimate PhenoAge [19], and DNAmTL [20]. DNAmMSS was developed as a proxy measure for exposure to maternal smoking during pregnancy by Rauschert et al. [17]. The score was developed with 204 CpGs using elastic net regression. It was first tested in the Raine Study using tenfold cross-validation and then validated independently in NFBC1986. PhenoAge was developed using 513 CpG sites from whole blood of adults [19]. It was trained on a chronological age-based composite clinical phenotypic measures of age, including nine biomarkers: albumin, creatinine, glucose, lymphocytes, C-Reactive Protein, mean cell volume, red cell distribution, alkaline phosphate, and white blood cell counts. The used intermediary measure was calculated as the residuals from the regression of DNAm age and chronological age. Among different measures of DNAm age scores, such as Hannum [25], Horvath [26], Horvath’s estimate for skin and blood [27], we included PhenoAge in our study as it was strongly correlated with chronological aging. PhenoAge is generated using different metabolic and aging markers, thus more closely representing biological health variance. DNAmTL is based on 140 CpGs and applicable over the entire age spectrum. It is considered more robust than Leucocyte TL and outperformed in detecting the association of age, sex, behavioral factors, with numerous clinical intermediary traits [20].
Adolescent outcomes
The adolescent measures were comparable in both cohorts, available at 16 years in NFBC1986 and at 17 years in the Raine Study. In both the studies, clinical examination was carried out to estimate the anthropometric and cardio-metabolic traits. Height, weight, and WC were measured by a nurse. Height and weight were converted to BMI as kg/m2 in the current study. BP was measured twice with ten minutes apart and the average of the measurements was used. Blood samples were extracted after overnight fasting to measure fasting glucose (mmol/l), insulin (mmol/l) and lipids (mmol/l) measures. The information on psychological symptoms was collected using Youth Self-Report scale [28]. In this study we included three sub-scales including questions that indicate anxious-depressed, withdrawn depressed and somatic complaints (supplementary methods, additional file 1).
Statistical analysis
Factor analysis
We employed a latent variable approach which is robust to measurement error and allows for variable reduction. Two different latent factor structures were generated: one for prenatal exposures and another for adolescent psycho-cardiometabolic intermediary traits. The prenatal latent factors were derived based on a previous study conducted in NFBC1986 [8]. Adolescent psycho-cardiometabolic latent factors were modeled independently in each cohort. We used Mplus 7.0 employing EFA to first identify the structure for adolescent psycho-cardiometabolic traits and confirmatory factor analysis (CFA) to confirm the final model for both prenatal and adolescent factors [29]. The analysis used weighted least squares mean and variance adjusted parameter estimates which is appropriate for categorical variables and geomin oblique rotations for correlations between the factors. The factorial structure was determined using model fit indices: RMSEA < 0.06, CFI > 0.90, and TLI > 0.90 [30, 31]. Factor scores were extracted (continuous values with mean = 0 and SD = 1) for each latent factor to use in the subsequent analysis.
Correlation matrix
We used Pearson correlation to identify the correlation matrix between the following variables: prenatal latent factors, adolescent psycho-cardiometabolic latent factors, DNAmMSS, PhenoAge and DNAmTL.
Structural equation modeling
Using AMOS v7 [32], all the measures were combined in a structural equation model (SEM) to investigate the proposed pathways in both cohorts separately. A common multimorbidity latent factor from the four adolescent psycho-cardiometabolic co-morbidities factors was created using second-order factor approach. This allowed us to look at the relationship of each of the components and to test our hypothesis that an interplay of adversities from early life are modulated through epigenetic markers leading to shared pathways to multimorbidity in adolescence. The prenatal latent factors included in this study have been tested previously for their relationship with birth weight. Hence, birth weight was not included in the model.
Results
The complete case sample sizes were 490 for NFBC1986 and 995 for the Raine Study (Table 1). In comparison to NFBC1986, the Raine Study mothers were more often unmarried/single/separated, nulliparous and their smoking and alcohol use were higher during pregnancy. Among adolescent measures, NFBC1986 adolescents had higher fasting glucose, insulin, and diastolic blood pressure and the Raine Study had higher BMI (Body Mass Index), WC (Waist Circumference), and triglycerides levels.
Latent factors
A similar three-factor structure supported by CFA with all measures loading strongly onto their respective prenatal latent factors in both cohorts (NFBC1986 = RMSEA (Root Mean Square Error of Approximation): 0.03, CFI (Comparative Fit Index): 0.98, TLI (Tucker Lewis Index): 0.97; Raine Study = RMSEA: 0.05, CFI: 0.98, TLI: 0.97) (Fig. 1). The first factor characterized by pre-and-end pregnancy BMI was labeled as ‘F1prenatal-BMI’. The second factor was labeled as ‘F2prenatal-Socioeconomic-Obstetric-Profile (SOP)’ representing ‘parity’, ‘maternal age’ and ‘unmarried status’. The third factor characterized maternal and paternal smoking and maternal alcohol use, termed as ‘F3prenatal-Lifestyle’. Similar correlations between factors were observed in both cohorts, with strongest correlation between ‘F2prenatal-SOP’ and ‘F3prenatal-lifestyle’.
Confirmatory factor analysis model of prenatal exposures in a NFBC1986 and b Raine Study. Boxes represent observed variables; circles represent latent factors and two-way arrows represent correlation between factors. Pearson correlation coefficients are written in italics. Values represent factor loadings of observed variables on latent factor. BMI body mass index, SOP socio-obstetric-profile
For adolescent psycho-cardiometabolic traits, EFA (Exploratory Factor Analysis) yielded four-factor structure displaying distinct biological and psychological groupings (Supplementary Table S1, Additional File 1). This was further supported by the CFA (Fig. 2) and the fit statistics, structures and factor loadings were comparable across both cohorts (NFBC1986 = RMSEA: 0.04, CFI: 0.98, TLI: 0.98; Raine Study = RMSEA: 0.06, CFI: 0.97, TLI: 0.96). The factors were labeled to closely represent the included observed variables, for instance: ‘F1adolescent-Anthropometrics’ to characterize BMI and WC, ‘F2adolescent-InsulinTG’ to characterize insulin and triglyceride, ‘F3adolescent-BP’ to characterize systolic and diastolic BP, and ‘F4adolescent-Mental health’ to characterize anxious-depressed, withdrawn depressed and somatic complaints. Across both cohorts, the strongest correlations among the latent factors were observed between ‘F1adolescent-Anthropometrics’ and ‘F2adolescent-InsulinTG’ (Fig. 2). Differences were observed in the correlations of ‘F4-Mental health’ with other factors between the two cohorts.
Confirmatory factor analysis model of adolescent comorbidities in a NFBC1986 and b Raine Study. Boxes represent observed variables; circles represent latent factors and two-way arrows represent correlation between factors. Pearson correlation coefficients are written in italics. Values represent factor loadings of observed variables on latent factor. BP blood pressure, TG triglycerides
Correlations between latent factors and epigenetic biomarkers
Distinct correlation clusters and similarities were observed between variables across both cohorts (Supplementary Fig S1, Additional File 1). Most of the variables were significantly correlated (P < 0.001). Among maternal factors, ‘F1prenatal-BMI’ was most strongly correlated with adolescent factors. For epigenetic markers, DNAmMSS showed strongest correlated with ‘F2prenatal-SOP’ and ‘F3prenatal-Lifestyle’, followed by DNAmTL which was inversely correlated with most of the factors with exception of ‘F1prenatal-BMI’, ‘F2prenatal-SOP’ and ‘F4adolescent-Mental health’.
SEM
An overview of the path model in accordance with our hypothesis is shown in Fig. 3, displaying comparable pathways in both cohorts. The fit indices for the multilevel SEM indicated a good model fit for both the cohorts (NFBC1986 = RMSEA: 0.03, CFI: 0.99, TLI: 0.98; Raine Study = RMSEA: 0.02, CFI: 0.99, TLI: 0.98). The psycho-cardiometabolic multimorbidity latent factor showed stronger representation of adolescent biological indicators than the mental health indicators. In the SEM pathways, stronger direct effects of the ‘F1prenatal-BMI’ (NFBC1986 = β: 0.27; Raine Study = β: 0.39) and ‘F2prenatal-SOP’ (β: −0.11) were observed on adolescent psycho-cardiometabolic multimorbidity factor. F3prenatal-Lifestyle showed only indirect effect (NFBC1986 = β: 0.04; Raine Study = β: 0.12 -Supplementary Table S2, Additional File 1) on the psycho-cardiometabolic multimorbidity factor. F3prenatal-Lifestyle had the strongest direct effect on the DNAmMSS (NFBC1986 = β: 0.36; Raine Study = β: 0.84). The indirect effect of the prenatal factors on multimorbidity through epigenetic markers was mediated from DNAmTL and DNAmMSS going through PhenoAge in Raine study (P < 0.05).
Structural equation modeling pathways in a NFBC1986 and b Raine Study. The values are standardized regression coefficients of direct effect. Values with P < 0.05 are denoted with bold fonts and P > 0.05 with normal font. BP blood pressure, BMI body mass index, DNAmMSS DNA methylation maternal Smoking Score, SOP socio-obstetric-profile, TG triglycerides, TL telomere length
Discussion
The current study is novel in providing an overview of the shared molecular pathways arising from in utero adversities on different psycho-cardiometabolic measures as a multimorbidity in adolescents. Importantly, we did a cross-cohort comparison of our findings from distinctly independent birth cohorts from two culturally diverse countries (Finland and Australia). We observed both direct and indirect effects of prenatal latent factors on adolescent psycho-cardiometabolic multimorbidity through composite epigenetic scores, displaying the importance of epigenomes on later health outcomes. Our objective was to further expand on the understanding of the structure of prenatal adversities and mental and physical health especially in adolescence which has been lacking from the existing literature.
We observed distinct characteristics between the two cohorts. Although, both cohorts were recruited at the same time point, maternal smoking and alcohol use was much higher in Raine compared to NFBC1986. Moreover, cardio-metabolic measurements varied between the two cohorts, indicating health differences between the two cohorts. In line with our previous findings [8], the prenatal latent factor model fitted the data well and revealed similar correlations between both cohorts. A distinct pattern was observed for adolescents’ psycho-cardiometabolic traits, showing separate biological and psychological groupings (Fig. 2), as seen in a previous study [33]. Importantly, similar structure and factor loadings were observed for both cohorts when constructed independently. While, biological patterns were quite comparable among each other, heterogeneity was observed between ‘F4adolescent-Mental health’ and its correlation with other factors across the cohorts. For NFBC1986, ‘F4adolescent-Mental health’ was largely represented by anxious-depressed measure, and it was negatively correlated with ‘F1adolescent-Anthropometric’ and ‘F3adolescent-BP’. On the other hand, in the Raine Study, ‘F4adolescent-Mental health’ was equally characterized by each psychological symptom subscale (anxious-depressed, withdrawal depressed and somatic complaints) and was positively correlated with other latent factors. This suggests that the biological parameters behave similarly between different populations but not psychological aspects. The reason may be that these patterns in adolescent period behave differently from adult patterns; attributable to their rapid hormonal changes, and wide range of biological, psychological and social challenges occurring in adolescence phase [34]. Additionally, these are culturally, socially and genetically different populations from different continents, where dynamics of perceiving health may also vary largely [35].
Our multimorbidity second-order factor sheds important insights on the relationship between and with psycho-cardiometabolic traits in its entirety. The factor structure was largely representative of biological measures and less of mental states. This was expected as the correlations between biological factors and mental states were weak in the CFA model. Despite the imbalance, it is very interesting to note that all the psycho-cardiometabolic comorbidity factors loaded into one factor that replicates between cohorts. Individuals with mental health problems have up to 14 years of shorter life expectancy, which is often partly accounted by the co-occurring physical diseases [36]. Moreover, heritability studies suggests that the causes of multimorbidity have both genetic and environmental components shared between physical and mental disorders [37, 38]. Therefore, it was worthwhile to unravel the shared relationships between psycho-cardiometabolic multimorbidity, which is not captured when looking at the traits individually.
Together, our SEMs revealed plausible pathways to multimorbidity. Specifically, among prenatal latent factors, ‘F1prenatal-BMI’ had the strongest direct influence on adolescent psycho-cardiometabolic multimorbidity. Maternal BMI embodies both a biological dimension as well as lifestyle and social factors, such as maternal age, marital status, smoking, and alcohol use [39]. These correlations were also reflected in our correlation matrix (Supplementary Fig S1, Additional File 1).
In the same way, ‘F2prenatal-SOP’ showed a direct effect on multimorbidity, but here the direction was negative, and no effect was modulated through epigenetic factors. This suggests that not all early life influences have epigenetic influence, particularly social factors (maternal age, marital status, parity). Additionally, the negative effect on the psycho-cardiometabolic multimorbidity factor highlights the protective dimension of social factors, such as decreased parity, younger maternal age, and married status. The ‘F3prenatal-Lifestyle’, while not showing a direct effect, showed a strong indirect effect in Raine study, primarily through DNAmMSS. Its strong intermediary role from ‘F3maternal-Lifestyle’ in our pathway analysis (Fig. 3) confirms the validity of the score as a proxy of ‘in utero adversity’ since it mirrored the known association of prenatal smoking-related epigenetic changes on cardio-metabolic health of the offspring in previous observational studies [13, 14, 16].
Epigenetic markers are important molecular readout of diverse environmental exposures across the lifespan. In our study, we observed that DNAmTL and DNAmMSS showed direct as well indirect influence going through PhenoAge marker. Increasing evidence supports the concept of molecular aging as a component of chronic diseases and an important tool for predicting biological age of an individual [18]. Biological age evaluated using these epigenetic markers has been shown to vary within individuals of same chronological age based on the incidence of chronic mental and physical diseases [40] and is also significantly influenced by intrauterine conditions [41]. DNAmTL showed negative relationship with all the path variables in our study. Telomere length is largely determined already during early fetal development and associates with several maternal factors during pregnancy, including maternal smoking, stress, socioeconomic status, BMI and gestational diabetes [42]. It is speculated that shorter TL may weaken the replicative potential and diminish somatic repair contributing to degenerative diseases such as cardio-metabolic diseases [43]. Correspondingly, our findings regarding PhenoAge were consistent with previous studies in showing the association with cardiometabolic risk factors. Importantly, it was observed to mediate the indirect effect of all the other epigenetic biomarker path factors particularly in Raine Study, highlighting its importance on phenotypic outcomes. Studies from both European and African American cohorts have reported association of PhenoAge with a wide range of phenotypes, such as smoking, blood pressure, insulin, glucose, triglycerides, and low-density lipid cholesterol [19, 44]. Nonetheless, PhenoAge is relatively new DNAm age estimate, so further replication is required to fully understand its association with a range of health outcomes. However, we observed in our study that more than 20% adolescents with personal smoking status, hence it could be a potential confounder in the relationship between epigenetic markers and psycho-cardiometabolic traits [45, 46].
A further point to note is that in both prenatal biopsychosocial and in adolescent psycho-cardiometabolic constructs, metabolic factor (F1maternal-BMI and F1adolescent-Anthropometric) showed consistently strongest correlations and largest representation in the latent factors. Thus, suggests that in our study adiposity, a potentially modifiable factor, had a predominant role over other predictors in defining adolescents’ health.
Strengths and limitations
This is the first study to use a factor structure approach to examine the latent relationship between prenatal adversities factors and later adolescent psycho-cardiometabolic health. The benefit of using a factor structure instead of an individual measure of biological health or psychological status is that it allows us to account for the different aspects of these variables, represented by the sub-factors of commonality [30]. A further advantage is that the magnitude of factor loadings is determined empirically and does not comply with the assumption that all component measures have equal weighting.
We acknowledge the limitations of this study. In NFBC1986 methylation, sample size was much smaller than the full cohort sample. However, the characteristics of both samples were relatively comparable (Supplementary Table S3, Additional File 1). We included the most closely related and easily accessible prenatal measures as it can be challenging to develop a comprehensive model with maximum available measures, for which, we had limited similar prenatal measures harmonized between both cohorts. Mental health measures used in the study are subjective in nature and self-reported and might be under-reported leading to potential biases. There are other DNAm age estimates, such as Horvath [26], Hannum [25], and Horvath’s estimate for skin and blood [27], developed to predict biological age. However, in our study, DNAmTL and PhenoAge were more closely related to phenotypic markers than other DNAm age estimates. Both these markers were also recently developed and suggested to correlate better with mortality and morbidity [19]. A common limitation of biological age estimates is that they rely on specific organs or tissue; however, PhenoAge has been observed to relate with the wider range of tissue and cell types than other markers [19]. DNA methylation is a dynamic process influenced by multiple social, environmental, and lifestyle factors throughout the life course. In our study, both DNA methylation and psycho-cardiometabolic traits are measured at the same time point i.e., 16–17 years. Therefore, we cannot assume temporality, and it is plausible that there is reverse causation or bidirectional association between them. The mediated path coefficients observed in our study are small. Our study includes adolescent populations which are generally healthy and therefore the findings from this study should be interpreted in the same context and cannot be generalized to later ages.
Conclusions
The present study exemplifies in two different cohorts similar composite structure of in utero maternal measures and psycho-cardiometabolic traits in adolescence, providing clarity on measures with cumulative risk. Our findings from cross-cohort analysis elucidate the differences in health between them and enhance the understanding of plausible common shared pathways from early life to psycho-cardiometabolic health through underlying epigenetic markers.
Data availability
NFBC data are available from the University of Oulu, Infrastructure for Population Studies. Permission to use the data can be applied for research purposes via electronic material request portal. In the use of data, we follow the EU general data protection regulation (679/2016) and Finnish Data Protection Act. The use of personal data is based on cohort participant’s written informed consent at his/her latest follow-up study, which may cause limitations to its use. Please, contact NFBC project center (NFBCprojectcenter@oulu.fi) and visit the cohort website (www.oulu.fi/nfbc) for more information.
References
Carney R, Firth J, Pedley R et al (2021) The clinical and behavioral cardiometabolic risk of children and young people on mental health inpatient units: a systematic review and meta-analysis. Gen Hosp Psychiatry 70:80–97. https://doi.org/10.1016/j.genhosppsych.2021.03.007
Cota BC, Priore SE, Ribeiro SAV et al (2021) Cardiometabolic risk in adolescents with normal weight obesity. Eur J Clin Nutr. https://doi.org/10.1038/s41430-021-01037-7
WHO adolescent development. https://www.who.int/maternal_child_adolescent/topics/adolescence/development/en/
Viner RM, Ozer EM, Denny S et al (2012) Adolescence and the social determinants of health. Lancet (London, England) 379:1641–1652. https://doi.org/10.1016/S0140-6736(12)60149-4
Hoffman S, Hatch MC (1996) Stress, social support and pregnancy outcome: a reassessment based on recent research. Paediatr Perinat Epidemiol 10:380–405. https://doi.org/10.1111/j.1365-3016.1996.tb00063.x
Kramer MS, Seguin L, Lydon J, Goulet L (2000) Socio-economic disparities in pregnancy outcome: why do the poor fare so poorly? Paediatr Perinat Epidemiol 14:194–210. https://doi.org/10.1046/j.1365-3016.2000.00266.x
Choudhary P, Ronkainen J, Nedelec R et al (2022) The relationship of life-course patterns of adiposity with type 2 diabetes, depression, and their comorbidity in the Northern Finland Birth Cohort 1966. Int J Obes 46:1470–1477. https://doi.org/10.1038/s41366-022-01134-y
Parmar P, Lowry E, Vehmeijer F, et al (2020) Understanding the cumulative risk of maternal prenatal biopsychosocial factors on birth weight: a DynaHEALTH study on two birth cohorts. J Epidemiol Community Health:jech-2019-213154. https://doi.org/10.1136/jech-2019-213154
Küpers LK, Monnereau C, Sharp GC et al (2019) Meta-analysis of epigenome-wide association studies in neonates reveals widespread differential DNA methylation associated with birthweight. Nat Commun 10:1893. https://doi.org/10.1038/s41467-019-09671-3
Robertson KD (2005) DNA methylation and human disease. Nat Rev Genet 6:597–610. https://doi.org/10.1038/nrg1655
Reynolds RM, Jacobsen GH, Drake AJ (2013) What is the evidence in humans that DNA methylation changes link events in utero and later life disease? Clin Endocrinol (Oxf) 78:814–822. https://doi.org/10.1111/cen.12164
Joehanes R, Just AC, Marioni RE et al (2016) Epigenetic signatures of cigarette smoking. Circ Cardiovasc Genet 9:436–447. https://doi.org/10.1161/CIRCGENETICS.116.001506
Parmar P, Lowry E, Cugliari G et al (2018) Association of maternal prenatal smoking GFI1-locus and cardio-metabolic phenotypes in 18,212 adults. EBioMedicine 38:206–216. https://doi.org/10.1016/j.ebiom.2018.10.066
Rauschert S, Melton PE, Burdge G, et al (2019) Maternal smoking during pregnancy induces persistent epigenetic changes into adolescence, independent of postnatal smoke exposure and is associated with cardiometabolic risk. Front Genet 10. https://doi.org/10.3389/fgene.2019.00770
Küpers LK, Xu X, Jankipersadsing SA et al (2015) DNA methylation mediates the effect of maternal smoking during pregnancy on birthweight of the offspring. Int J Epidemiol 44:1224–1237. https://doi.org/10.1093/ije/dyv048
Wiklund P, Karhunen V, Richmond RC et al (2019) DNA methylation links prenatal smoking exposure to later life health outcomes in offspring. Clin Epigenetics 11:97. https://doi.org/10.1186/s13148-019-0683-4
Rauschert S, Melton PE, Heiskala A et al (2020) Machine learning-based DNA methylation score for fetal exposure to maternal smoking: development and validation in samples collected from adolescents and adults. Environ Health Perspect 128:097003. https://doi.org/10.1289/EHP6076
Costantino S, Mohammed SA, Ambrosini S, Paneni F (2019) Epigenetic processing in cardiometabolic disease. Atherosclerosis 281:150–158. https://doi.org/10.1016/j.atherosclerosis.2018.09.029
Levine ME, Lu AT, Quach A, et al (2018) An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY) 10:573–591. https://doi.org/10.18632/aging.101414
Lu AT, Seeboth A, Tsai P-C, et al (2019) DNA methylation-based estimator of telomere length. Aging (Albany NY) 11:5895–5923. https://doi.org/10.18632/aging.102173
Järvelin MR, Hartikainen-Sorri A-L, Rantakallio P (1993) Labour induction policy in hospitals of different levels of specialisation. BJOG An Int J Obstet Gynaecol 100:310–315. https://doi.org/10.1111/j.1471-0528.1993.tb12971.x
Newnham JP, Evans SF, Michael CA et al (1993) Effects of frequent ultrasound during pregnancy: a randomised controlled trial. Lancet 342:887–891. https://doi.org/10.1016/0140-6736(93)91944-H
White SW, Eastwood PR, Straker LM et al (2017) The Raine study had no evidence of significant perinatal selection bias after two decades of follow up: a longitudinal pregnancy cohort study. BMC Pregnancy Childbirth 17:207. https://doi.org/10.1186/s12884-017-1391-8
Huang RC, Melton PE, Burton MA et al (2022) Adiposity associated DNA methylation signatures in adolescents are related to leptin and perinatal factors. Epigenetics 17:819–836. https://doi.org/10.1080/15592294.2021.1876297
Hannum G, Guinney J, Zhao L et al (2013) Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell 49:359–367. https://doi.org/10.1016/j.molcel.2012.10.016
Horvath S (2013) DNA methylation age of human tissues and cell types. Genome Biol 14:R115. https://doi.org/10.1186/gb-2013-14-10-r115
Horvath S, Oshima J, Martin GM et al (2018) Epigenetic clock for skin and blood cells applied to Hutchinson Gilford Progeria Syndrome and ex vivo studies. Aging (Albany NY) 10:1758–1775. https://doi.org/10.18632/aging.101508
Achenbach TM, Dumenci L, Rescorla LA (2001) Ratings of relations between DSM-IV diagnostic categories and items of the CBCL/6-18, TRF, and YSR. University of Vermont
Muthén LK, Muthén BO (1998–2015) Mplus user’s guide, 7th edn. Muthén & Muthén, Los Angeles, CA
Kline RB (2015) Principles and practice of structural equation modeling, 4th ed. Guilford Press, New York, NY
Hu L, Bentler PM (1999) Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Model A Multidiscip J 6:1–55. https://doi.org/10.1080/10705519909540118
Arbuckle JL (2006) Amos (Version 7.0)
Lowry E, Rautio N, Karhunen V et al (2019) Understanding the complexity of glycaemic health: systematic bio-psychosocial modelling of fasting glucose in middle-age adults; a DynaHEALTH study. Int J Obes 43:1181–1192. https://doi.org/10.1038/s41366-018-0175-1
Sawyer SM, Azzopardi PS, Wickremarathne D, Patton GC (2018) The age of adolescence. Lancet Child Adolesc Heal 2:223–228. https://doi.org/10.1016/S2352-4642(18)30022-1
Shah R, Hagell A, Cheung R (2019) 5 international comparisons of health and wellbeing in adolescence and early adulthood. Research report February. Nuffield Trust ISBN: 978-1-910953-62-4
Plana-Ripoll O, Pedersen CB, Agerbo E et al (2019) A comprehensive analysis of mortality-related health metrics associated with mental disorders: a nationwide, register-based cohort study. Lancet 394:1827–1835. https://doi.org/10.1016/S0140-6736(19)32316-5
Wray NR, Ripke S, Mattheisen M et al (2018) Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet 50:668–681. https://doi.org/10.1038/s41588-018-0090-3
Demontis D, Walters RK, Martin J et al (2019) Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet 51:63–75. https://doi.org/10.1038/s41588-018-0269-7
Marcellini F, Giuli C, Papa R et al (2009) Obesity and body mass index (BMI) in relation to life-style and psycho-social aspects. Arch Gerontol Geriatr 49:195–206. https://doi.org/10.1016/j.archger.2009.09.029
Huang R-C, Lillycrop KA, Beilin LJ et al (2019) Epigenetic age acceleration in adolescence associates with BMI, inflammation, and risk score for middle age cardiovascular disease. J Clin Endocrinol Metab 104:3012–3024. https://doi.org/10.1210/jc.2018-02076
Bergsma T, Rogaeva E (2020) DNA methylation clocks and their predictive capacity for aging phenotypes and healthspan. Neurosci insights 15:2633105520942221. https://doi.org/10.1177/2633105520942221
Hjort L, Vryer R, Grunnet LG et al (2018) Telomere length is reduced in 9- to 16-year-old girls exposed to gestational diabetes in utero. Diabetologia 61:870–880. https://doi.org/10.1007/s00125-018-4549-7
Aviv A, Shay JW (2018) Reflections on telomere dynamics and ageing-related diseases in humans. Philos Trans R Soc B Biol Sci 373:20160436. https://doi.org/10.1098/rstb.2016.0436
Ammous F, Zhao W, Ratliff SM et al (2021) Epigenetic age acceleration is associated with cardiometabolic risk factors and clinical cardiovascular disease risk scores in African Americans. Clin Epigenetics 13:55. https://doi.org/10.1186/s13148-021-01035-3
Weitzman M, Cook S, Auinger P et al (2005) Tobacco smoke exposure is associated with the metabolic syndrome in adolescents. Circulation 112:862–869. https://doi.org/10.1161/CIRCULATIONAHA.104.520650
Prince C, Hammerton G, Taylor AE et al (2019) Investigating the impact of cigarette smoking behaviours on DNA methylation patterns in adolescence. Hum Mol Genet 28:155–165. https://doi.org/10.1093/hmg/ddy316
Acknowledgements
We thank all cohort members and researchers who have participated in the study. We also wish to acknowledge the work of the NFBC project center.
The authors thank the Raine Study participants and their families, the Raine Study Team for cohort coordination and data collection, The NHMRC for their long-term contribution to funding the study over the last 25 years, and The Telethon Kids Institute for long-term support of the Raine Study. We also acknowledge The University of Western Australia, Curtin University, Women and Infants Research Foundation, Edith Cowan University, Murdoch University, The University of Notre Dame Australia, and the Raine Medical Research Foundation for providing funding for core management of the Raine Study.
Funding
Open Access funding provided by University of Oulu (including Oulu University Hospital). This work was supported by the European Union’s Horizon 2020 research and innovation program under grant agreement no. 733206 (LifeCycle), grant agreement no. 848158 (EarlyCause), grant agreement no. 873749 (LongITools) and the University of Oulu and the Research Council of Finland project 326291. NFBC1986 study was supported by EU QLG1-CT-2000–01643 (EUROBLCS) Grant no. E51560, NorFA Grant no. 731, 20056, 30167, USA / NIH 2000 G DF682 Grant no. 50945.
The Raine Study team has been supported by NHMRC (Beilin et al., ID 403981; Eastwood et al., ID 1027449; Huang et al., ID 1059711; Palmer et al., ID 572613;), NHMRC Program Grant (Stanley et al., ID 353514), Canadian Institutes of Health Research—CIHR (Lye et al., MOP-82893), The Centre for Sleep Science, School of Anatomy, Physiology & Human Biology, The University of Western Australia, and the Raine Medical Research Foundation for cohort co-ordination and data collection. The epigenetic data are collected through NHMRC Huang et al., ID 1059711. RCH is supported by NHMRC ID1053384 and ID733206. AL is supported by an NHMRC Emerging Leader Fellowship ID 2010063. JC is supported by the National Health and Medical Research Council, Australia grant ID GNT114285.
The funding bodies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
PC, SS, and RCH conceptualized and designed the study. P.C. analyzed the data and wrote the first draft. SS, RCH, PC, PM, JC, JR, AL, VK, JM, and MRJ contributed to the study design, to the interpretation of the results, edited and approved the final version of the manuscript and agreed to accountable to accuracy and integrity of the study. SS, RCH, and PC are the guarantors of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethics approval and consent to participate
For the NFBC1986, the written informed consents were given by parents and adolescents and studies were approved by the ethics committee of the Northern Ostrobothnia Hospital District in Oulu (EETTMK: 108/2017) and Oulu University, Faculty of Medicine, Oulu, Finland. For Raine study, informed and written consent was provided by the parents or care givers and adolescents. Ethics approval was obtained from the University of Western Australia (approval numbers: RA/4/1/6613, 1214-EP, RA-4-1-2646).
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Choudhary, P., Ronkainen, J., Carson, J. et al. Developmental origins of psycho-cardiometabolic multimorbidity in adolescence and their underlying pathways through methylation markers: a two-cohort study. Eur Child Adolesc Psychiatry 33, 3157–3167 (2024). https://doi.org/10.1007/s00787-024-02390-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00787-024-02390-1