Abstract
This study investigates early onset of treatment response as predictor of symptomatic and functional outcome 3 years after initiation of methylphenidate (MPH) administration in a naturalistic, clinical cohort of children and adolescents with ADHD. Children were followed across an initial 12-week MPH treatment trial and after 3 years, with ratings of symptoms and impairment. Associations between a clinically significant MPH treatment response in week 3 (defined as ≥ 20% reduction in clinician-rated symptoms) and in week 12 (defined as ≥ 40% reduction), and 3-year outcome were tested in multivariate linear regression models, adjusting for sex, age, comorbidity, IQ, maternal education, parental psychiatric disorder, and baseline symptoms and function. We did not have information on treatment adherence or the nature of treatments beyond 12 weeks. 148 children, mean age 12.4 years (range 10–16 years), 77% males, participated in the follow-up. We found a significant decrease in symptom score from baseline [M = 41.9 (SD = 13.2)] to 3-year follow-up [M = 27.5 (SD = 12.7), p < 0.001, and in impairment score from baseline (M = 41.6 (SD = 19.4)] to 3-year follow-up [M = 35.6 (SD = 20.2), p = 0.005]. Treatment responses in week 3 and week 12 were significant predictors of the long-term outcome of symptoms, but not of impairment at 3-year follow-up, when adjusting for other well-known predictors. Early treatment response predicts long-term outcome over and above other well-known predictors. Clinicians should follow-up patients carefully, during the first months of treatment, and detect non-responders, since there might be a window of opportunity to alter the outcome, by changing the treatment strategy.
Clinical trial registration: ClinicalTrials.gov, registration number NCT04366609, April 28, 2020 retrospectively registered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methylphenidate (MPH) is the first-line medication [11] in treatment of ADHD in children and adolescents. The evidence for the short-term effect is well established, based on randomized controlled trials (RCT’s). In contrast, only weak evidence exists concerning its long-term effect, due to ethical and methodological challenges in conducting long-term RCTs. Register and cohort studies using a within-individual design combine information of ADHD medication use, incidents of risk- and suicidal behavior, and academic performance during medicated and nonmedicated periods, within the same individual. These studies suggest a decreased risk during medicated periods regarding accidents, suicidal behavior, substance abuse, and delinquency [9, 12, 22, 27, 29], and a positive effect on student grade point average [17]. In spite of this, the adult follow-up in the Multimodal Treatment of Attention Deficit Hyperactivity (MTA) Study [31], and other follow-up studies in late adolescence [30, 33] have demonstrated that the long-term outcome in childhood ADHD is independent of treatment with ADHD medication during the follow-up period. Moreover, the long-term compliance to ADHD medication is weak [6, 31], with perceived effectiveness of ADHD medication and systematic titration of medication being some of the factors associated with a better compliance to medication [16]. A few short-term studies have investigated predictors of the immediate effect of treatment with MPH and reported that higher baseline severity of inattention and higher response time variability on continuous performance tests were associated with a poorer response to medication with MPH, measured as mean composite score on the Continuous Performance Test [26] and as total score on the Korean ADHD rating scale [21].
Early treatment response as predictor was studied in a double-blind placebo controlled trial of MPH treatment in children with ADHD [7] which described that a positive behavioral change after the first single dose of MPH was the strongest predictor of improvement in multiple settings after 4 weeks of treatment.
Since adherence to ADHD medication is weak, it is difficult to disentangle predictors of long-term outcome of the disorder from predictors of long-term treatment effects in ADHD. Increased severity of ADHD symptoms in childhood, psychiatric comorbidity, and family adversities have consistently been identified as predictors of a poorer outcome of childhood ADHD. Higher levels of parent-reported impairment in daily and social functioning, female sex, and lower IQ of the child have also been associated with poorer long-term outcomes [8, 10, 20, 28, 33].
This study aimed to extend the evidence base for long-term symptomatic and functional outcome of MPH treatment, and the predictors of outcomes focusing on the early treatment response as predictor in 7–12-year-old children with a recent diagnosis of ADHD and first initiation of treatment with MPH. The association between the markers of early response and the long-term outcomes were adjusted for potential confounders. Our overarching aim was to investigate the potential for clinical use of instrument-based assessments and evaluation of the early and individual course of response to MPH treatment, as a strategy for improved short- and long-term outcomes in clinical care. More specifically, we tested if an early treatment response versus non-response to MPH predicted the long-term outcome measured as ADHD symptoms and level of daily functioning.
We tested the following hypotheses:
-
(a)
The early treatment response, measured as reduction in symptoms at 3 weeks and 12 weeks, would be associated with the symptomatic outcome after 3 years.
-
(b)
The early treatment response, measured as reduction in symptoms at 3 weeks and 12 weeks, would be associated with the functional outcome after 3 years.
-
(c)
The associations would be robust after adjustment for known predictors (gender, age, comorbidity, IQ, maternal educational level, and parental psychiatric disorders), and for baseline level of symptoms and baseline level of impairment.
Methods
Participants
The study is a follow-up of participants in the INDICES study, a prospective longitudinal 12-week ecologically valid observational study of first treatment with methylphenidate in a representative clinical sample of drug naïve children [19]. The study was conducted as part of the routine care in the clinic, and the individually monitored treatment with MPH was part of the study by the INDICES consortium aiming at personalizing the treatment of drugs metabolized by CES1 [5]. Patients were recruited from the Child and Adolescent Mental Health Centre, Mental Health Services, Capital Region of Denmark from August 2011 to December 2014. Participants at baseline included a total of 207 children (75.4% boys), aged 7–12 years (mean age 9.58 years), with a recent ICD-10 diagnosis of hyperkinetic disorder (F90.0–90.9), or attention-deficit disorder without hyperactivity (F98.8), IQ ≥ 70, and clinical indication for treatment with IR-MPH. The exclusion criteria were former treatment with any ADHD medication (MPH, dexamfetamine, lisdexamfetamine, or atomoxetine), contraindication for treatment with MPH, Danish language at a level that did not allow a valid exploration, or lack of informed consent.
For the 3-year follow-up, the parents and/or legal guardians of all 207 participant at baseline were contacted by letter, or later by telephone if they did not respond. They were informed of the study and invited to participate in the 3-year follow-up, by answering two questionnaires included in the contact letter. Only participants with data at baseline, week 3, week 12, and after 3 years are included in the analyses.
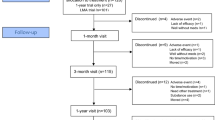
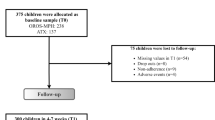
Figure 1 shows the flowchart of participants from baseline to the 3-year follow-up.
The 12-week MPH treatment trial and assessment procedures
Participants received an initial Immediate Release (IR) MPH dose (2.5/5 mg) based on bodyweight (< / > 30 kg), two or three times a day according to participants individual symptoms and needs. The dosing was individually titrated based on weekly assessments of effect and adverse reactions (AR), until AR prohibited further MPH dose increase, or cut-off for the normal range or borderline range on the ADHD-Rating Scale (ADHD-RS) was achieved. ADHD-RS has been validated in a Danish general population-based sample (n = 865 children) [32]. We used the standardized scores (t-scores) stratified for each sex and age group (7–9 and 10–12 years) to delineate the cut-off for the normal range (≤ 60 t-scores) or borderline range (60–70 t-scores) on the clinician-rated ADHD-RS-Clinician (ADHD-RS-C) total score (inattention plus hyperactivity–impulsivity scores) and scores of inattention and hyperactivity–impulsivity respectively, in the present study [13, 32, 37]. The study had a pre-defined MPH maximum dose of 2.1 mg/kg/day. The mean IR-MPH end-dose after 12 weeks was 1.0 (0.3), (range 0–1.79) mg/kg day.
During the trial, participants were assessed weekly, with ratings of ADHD symptoms (ADHD-RS-C) and AR’s on the Barkley’s Stimulant Side Effect Rating Scale (BSSERS) [4] conducted by the clinical investigator and the child’s regular clinician, based on telephone interviews with the parents, and clinical assessments at baseline, week 4, 8, and 12. These included physical examination, observation of the child, interview with the parents, and consensus ratings on the ADHD-RS-C. ADHD and disruptive behavior symptoms were rated by parents at baseline, and at 12-week follow-up, on the ADHD-Rating Scale Barkley version (ADHD-RS) [3]. Impairment in daily life- and social functioning was rated by parents on the Weiss Functional Impairment Rating Scale (WFIRS-P) [34] at baseline and at 12-week follow-up.
The 3-year follow-up
Three years after completing the 12-week MPH treatment trial, long-term outcome was evaluated by the parent-reported severity of ADHD and disruptive behavior symptoms, and impairment in daily life- and social functioning, using the ADHD-RS and the WFIRS-P questionnaires. We did not have information concerning use of ADHD medication, non-medical treatments, and adherence to treatment from week 12 to 3-year follow-up.
Outcome variables
The ADHD-RS-C consists of 18 clinician-rated questions evaluated on a four-point Likert scale from 0 (none = never or rarely) to 3 (severe = very often). It measures ADHD symptoms including a total score and two subscales: inattention (9 items) and hyperactivity–impulsivity (9 items). Thus, the total score ranges from 0 to 54. Higher scores indicate worse outcomes.
The ADHD-RS Barkley version is like the ADHD-RS-C but includes a behavior subscale. It consists of 26 parent-rated questions. In addition to the inattention- and hyperactivity–impulsivity subscales, it includes a disruptive behavior subscale (8 items). Thus, the total score ranges from 0 to 78. Higher scores indicate worse outcomes.
The WFIRS-P measures children's daily life- and social functioning. It consists of 50 parent-rated questions evaluated on a four-point Likert scale from 0 (never or not at all) to 3 (very often or very much). WFIRS-P covers six different domains: family (10 items); learning and school (10 items); activities of daily living (10 items); self-concept (3 items); social activities (7 items); and risky activities (10 items). Thus, the total score ranges from 0 to 150. Higher scores indicate worse outcomes. WFIRS-P has been validated in multiple cultures [35], including a recent Norwegian validation study [15].
Predictor variables
Treatment response after 3 weeks was defined as ≥ 20% reduction in clinician-rated ADHD-RS-C total score, between baseline and week 3. Treatment response after 12 weeks was defined as ≥ 40% reduction in clinician-rated ADHD-RS-C total score between baseline and week 12.
In addition, we included sex, age group divided in young (7–9 years) and old (10–12 years), comorbidity (none versus ≥ 1 comorbid diagnosis), IQ (full scale IQ 70–85/ > 85), and parental psychiatric disorder (none/ any maternal or paternal disorder). As a proxy measure of socio-economic status, we used maternal education.
Information of any parental lifetime psychiatric diagnoses was obtained from the Danish Psychiatric Central Register. Information of mother’s educational level was obtained at baseline by interview with the parent and categorized in three groups ranging from primary school to a longer higher education at university level, based on years of schooling and education (primary/lower secondary education, upper secondary education, higher education).
Ethics
The study was registered in ClinicalTrials.gov (NCT04366609). The study was approved by the Danish Data Protection Agency (P-2019-851). The Local Committee on Health Research Ethics was consulted (J.nr. H-B-2009-026) in accordance with national guidelines and the Declaration of Helsinki, and the study was evaluated not to be within their jurisdiction due to study design as an observational study. Participation was voluntary and data were kept confidential. The participants could withdraw their consent at any time without having to give reasons and with no consequences for their further treatment options.
Statistical analyses
Attrition analyses were performed to explore potential differences in baseline characteristics, ADHD-RS-C-, ADHD-RS- and WFIRS-P scores in the 12-week treatment trial between participants in the 3-year follow-up and those who were lost to follow-up. We used Chi-square tests for categorical variables, Mann–Whitney test for ordinal variables, and independent t tests for continuous variables.
Missing data on any items were not allowed on ADHD-RS-C and ADHD-RS. Ten percent missing data on items on WFIRS-P subscales were allowed, and missing data on items were set as 0 (never or not at all).
Paired-samples t tests were performed to explore the development of ADHD-RS and WFIRS-P scores from baseline, after 12 weeks of MPH treatment to 3-year follow-up.
Two-sample t tests were applied to test associations between a positive MPH treatment response in week 3 (≥ 20% reduction in symptom score) and in week 12 (≥ 40% reduction in symptom score), respectively, and outcome on the parent-rated ADHD-RS total score and WFIRS-P total score after 3 years.
Multiple linear regression analyses were conducted to test the effect of adjusting the crude associations obtained with two-sample t tests, for the selected predictor variables. Quantile plots [36] of the outcome variables in these multiple linear regression analyses (ADHD-RS total- and three subscales scores and WFIRS-P total score) were performed to assess the assumption of normally distributed outcomes. They showed approximately normal distribution for all variables. To explore the effect of including either treatment response at week 3, or treatment response at week 12, we tested two models of predictors. Model 1 includes sex, age group, comorbidity, IQ (IQ 70–85 vs > 85), maternal education, parental psychiatric disorder, baseline ADHD-RS total score, baseline WFIRS-P total score, and responder status in week 3. Model 2 includes all the same co-variates and responder status at week 12. The reason for including two models of predictors was to explore whether treatment response could be evaluated as early as after 3 weeks. The primary outcomes were ADHD-RS total score and WFIRS-P total score. In addition, we measured outcome on the three ADHD-RS subscales. Adjusted R2 was used as a measure of the proportion of the variance in the outcome variables, explained by potential predictors in the two models.
All analyses were conducted using the IBM SPSS Statistics 25 program. All tests were two sided, and significance was set at p < 0.05.
Results
Study population
Table 1 shows the baseline characteristics of the participants in the 3-year follow-up and those who dropped out. Of the original sample (n = 207) 148 had ADHD-RS-C ratings at baseline, week-3, and week-12, thus constituting the study population and a retention rate of 71%. WFIRS-P rating was available for 139 participants at baseline, and 132 at 3-year follow-up. Independent t tests, Mann–Whitney test, and Chi-square tests showed no significant differences in individual characteristics, comorbidities, parental psychiatric disorders, maternal education, ADHD subtypes, and scores on the ADHD-RS-C, ADHD-RS, and WFIRS-P between the 3-year follow-up sample and dropouts.
The course of ADHD symptoms and impairment in daily life- and social functioning over time
ADHD symptoms, measured by the ADHD-RS total score, decreased significantly from baseline [M = 41.9 (SD = 13.2)] to 12-week follow-up [M = 23.7(SD = 10.9), p < 0.001], followed by a slight but significant increase in score at 3-year follow-up [M = 27.5(SD = 12.7), p < 0.001].
Impairment in daily life and social functioning, measured by WFIRS-P total score, decreased significantly from baseline [M = 41.6 (SD = 19.4)] to 12-week follow-up [M = 29.4 (SD = 14.5), p < 0.001], indicating better functioning, followed by a slight but significant increase in score at 3-year follow-up [M = 35.6 (SD = 20.2), p = 0.005].
Early treatment response as predictor of long-term outcome (unadjusted analyses)
Analyses of the association between treatment response at 3 weeks (≥ 20% reduction in ADHD-RS-C total score) and 12 weeks (≥ 40% reduction in ADHD-RS-C total score), and symptomatic and functional outcome at 3-year follow-up showed the following results: week-3 responders (N = 56) had a significantly lower ADHD-RS-total score at 3-year follow-up [M = 24.1 (SD = 11.0)], compared with week-3 non-responders [M = 29.6 (SD = 13.3), t(146) = 2.632, p = 0.009]. Additionally, responders at week 12 (N = 121) had a significantly lower ADHD-RS-total score [M = 26.0 (SD = 12.3)] compared with week-12 non-responders [M = 34.2 (SD = 12.5) t(146) = 3.097, p = 0.002] at 3-year follow-up. There was no significant difference between week-3 responders [M = 31.9 (SD = 19.1)] and non-responders [M = 37.9 (SD = 20.6), t(143) = 1.736, p = 0.085) on daily and social functioning measured with the WFIRS-P total score at 3-year follow-up. Week-12 responders, however, had a significantly lower WFIRS-P total score [M = 33.9 (SD = 20.1) compared to week 12-non-responders (M = 43.3 (SD = 18.7), t(143) = 2.185, p = 0.031] at 3-year follow-up, indicating better functioning.
Adjusting for known predictors
Table 2 shows the results of the multivariate linear regression analyses of correlates of the ADHD-RS-total score outcome at the 3-year follow-up, adjusting the crude associations with the selected predictors in the two models, investigating the effects of the early treatment response after 3 or 12 weeks, respectively. Both remained significant predictors for better outcome after the adjustment. Female sex and young age (both models), higher baseline WFIRS-P score in model 1, and ≥ 1 comorbid diagnosis in model 2 significantly predicted worse outcome at 3 years (model 1 adj. R2 = 0.26, model 2 adj. R2 = 0.27).
Regarding the outcome measured on the ADHD-RS subscales (Online Resource Table 2b–2d), we found no significant associations of the treatment response in week 3 or week 12 with scores of inattention at the 3-year follow-up. Young age in both models, along with higher baseline WFIRS-P score in model 1, significantly predicted worse outcome of inattention at 3 years (model 1 adj. R2 = 0.08, model 2 adj. R2 = 0.09).
Regarding the outcome measured on the hyperactivity/ impulsivity subscale, the treatment response in week 3 and week 12 remained significant predictors for better outcome at 3 years. Female sex, young age, ≥ 1 comorbid diagnosis, and higher baseline ADHD-RS total score significantly predicted worse outcome of hyperactivity/impulsivity (model 1 adj. R2 = 0.23, model 2 adj. R2 = 0.22).
For the outcome measured on the disruptive behavior subscale, the treatment response in week 12 remained a significant predictor for better outcome at 3 years, whereas there was no significant effect of the treatment response in week 3. Higher baseline ADHD-RS total score and higher baseline WFIRS-P score significantly predicted worse outcome in both models, (model 1 adj. R2 = 0.25, model 2 adj. R2 = 0.26).
Regarding the functional outcome measured as the WFIRS-P total score at 3 years (Online Resource Table 2e), neither treatment response in week 3 nor week 12 reached significance. Higher baseline WFIRS-P total score was the only significant predictor (worse outcome) (model 1 adj. R2 = 0.17, model 2 adj. R2 = 0.16).
Discussion
In this 3-year follow-up study of a naturalistic, clinical cohort of children with ADHD, we studied the early response versus non-response to medical treatment with MPH as putative predictors for a better symptomatic and functional outcome 3 years after initiation of treatment, adjusting for other known predictors of the long-term outcome of treatment for ADHD. In summary, positive response to MPH treatment 3 and 12 weeks after initiation, measured respectively as a 20% or 40% reduction in symptoms, significantly predicted less hyperactivity/impulsivity and oppositional defiant symptoms at 3-year follow-up, over and above baseline symptoms and impairment, comorbidity, and other well-known predictors.
There may be several reasons why early treatment response can predict severity of disorder 3 years later. We did not have information of adherence to ADHD medication beyond 12 weeks in our study, but expectedly effective control of ADHD symptoms will result in less negative feedback from the environment, a better self-esteem, less peer rejection, and a better performance in school [2, 17, 23, 25]. Additionally, effective symptom control in the child will have a positive impact on family-life and parent child relationship [14, 18]. The experience of treatment failure may lead to negative expectations in the young person and the family toward medical treatment and the health-care system, resulting in non-compliance with medication and abandoned treatment in general. Contrary, an early positive treatment response may improve the working alliance between the child with ADHD, the family, and the clinician. Expectedly, this will enhance adherence to non-medical and medical treatment and support, and result in a better long-term outcome [16], which may be reflected by the results of our study. The reason could also be that there are other unknown factors associated with treatment response, that characterize aspects of ADHD or signals severity, which are not captured by the ADHD-RS scores, even after adjusting for well-known predictors.
The well-known predictors, such as sex, age, comorbidity, and severity of baseline symptoms and function, were treated as co-variates in the multivariate linear regression analyses, and most factors remained predictive, in line with other studies [10, 20, 33], whereas neither maternal educational level nor parental psychopathology were significant predictors of the outcome of the child in this treatment study. In their 6-year follow-up study, van Lieshout et al. [33] described the same result for parental educational level but showed that parental ADHD was a significant predictor. The reason for this difference could be that they used self-reported data on parental ADHD status based on the K-SADS interview, whereas we use register data, based on hospital contacts, and thus deal with more severe and less-frequent cases of parental psychopathology, which could have decreased its predictive ability. Both parental psychopathology and lower maternal education were slightly, but insignificantly more frequent in dropouts versus participants in our study, and autism spectrum disorders were more common in the group of participants compared to dropouts, though the difference was not significant. The rate of externalizing disorders was low in both participants and dropouts. This may influence the long-term outcome in the cohort and is important to notice when comparing with other studies [20, 28].
Our models of predictive variables explained as much as 27%, 23%, and 26% of the variance in 3-year outcome measured as ADHD-RS total score, hyperactivity/impulsivity score, and disruptive behavior score, respectively. Contrary to this, the predictive variables only explained up to 9% of the variance in ADHD-RS inattention score, and 17% of the variance in WFIRS total score, indicating that development of inattention and impairment in function are predicted by other factors than those under investigation in this study.
The significant decrease in severity of ADHD symptoms and impairment during the initial 12-week treatment trial, followed by a slight increase of both symptoms and impairment from week-12 to 3-year follow-up, may be explained by the fact that the systematic assessments and individually adapted medication in the trial stopped at week 12, leading to significantly less-frequent clinical follow-up evaluations of beneficial and adverse effects of medications, and daily life- and social functioning. Still, we found an overall significant decrease in symptoms and impairment from baseline to 3-year follow-up. This is in line with other long-term follow-up studies of childhood ADHD [20, 30, 33] describing clinical improvement over the course of adolescence regardless of treatment status. Participants in the study by van Lieshout et al. [33] were older at follow-up and had a larger reduction in hyperactivity–impulsivity symptoms, but the relative change scores of ADHD symptoms are difficult to compare due to differences in age, and follow-up period.
Our results indicate that clinicians must assess and treat those who do not respond to ADHD medication during the first months of treatment with bigger efforts, since they have a worse long-term prognosis, and the poorer prognosis was only partly explained by psychiatric comorbidity and other well-known predictors. If non-responders are detected early and addressed by evaluating the treatment strategy, there might be a window of opportunity to improve the long-term outcome. In cases of non-response, the clinician must consider whether the dosing or choice of ADHD medication should be altered, and whether the psychosocial support and treatment is sufficient. In cases with persistent non-response, a diagnostic re-evaluation must be considered. Children who respond well to ADHD medication should be regularly assessed according to the clinical guidelines [1], including assessment of beneficial- and side-effects, daily function in school, home and with peers, and adjustment of medical and non-medical treatment as needed.
Our results extend the evidence from the 8-year follow-up study of the MTA cohort, suggesting that the initial response to treatment is associated with a better long-term prognosis even after adjustment for the initial clinical presentation [24].
Strengths and limitations
The main strength of this study is the 3-year follow-up of a naturalistic clinical cohort, who represent a typical population of children with ADHD and one or more comorbid disorders, treated in the Child and Adolescent Mental Health Services in Denmark. The diagnosis of ADHD and comorbidities was based on best practice and standardized instruments including the K-SADS diagnostic interview. Associations of early treatment response and 3-year outcome were adjusted for other well-known predictors in multivariate linear regression analyses.
The study has several limitations. First, we do not have any test of interrater reliability of the diagnostic assessments. Incorrect diagnoses will have implications for the 3-year outcome. Second, the observational design without a control group makes it uncertain whether the observed significant decline in ADHD symptoms during the initial 12-week treatment trial was an effect of the medication or other factors, including the weekly contact with the clinical investigator, and whether the development of symptoms and impairment at 3-year follow-up just followed the natural course of the disorder. Third, all ratings of ADHD symptoms and function were not blinded. Clinicians and parents may have been biased by their wishes and efforts to improve the child’s symptoms and functioning. Fourth, we have no data concerning use of ADHD medication and non-medical treatments from week-12 to 3-year follow-up. Fifth, ratings of symptoms and functioning at 3-year follow-up did not consider treatment status, other psychiatric symptoms, and physical health. Sixth, we only have follow-up data from one time point during 3 years. Seventh, the huge methodological variability in studies investigating the long-term outcome in clinical cohorts of children with ADHD makes it difficult to compare the results of our study with other studies.
To get a better understanding of predictors and outcome in childhood ADHD, researchers should conduct long-term randomized controlled trials of treatments in representative clinical populations of children and adolescents with ADHD, to investigate the beneficial and adverse effects of different treatment approaches, adherence, and predictors of treatment outcome. Future naturalistic studies should investigate larger cohorts and, as it is possible in Denmark and other Scandinavian countries, include information from national registries such as the extent of psychosocial treatment, medication use, school graduation exams, and lifetime psychiatric and somatic diagnoses.
Conclusion
This 3-year follow-up of a clinical cohort of children with ADHD, without information of adherence to ADHD medication beyond 3 months, demonstrates that early treatment response at 3 and 12 weeks predicts a better long-term outcome. An early response to medical treatment after 3 weeks is a good prognostic factor, but some of the non-responders will respond at 12 weeks, suggesting that the treatment with MPH needs to be closely monitored to optimize treatment effect, including considering a switch to other medication, if MPH treatment is suboptimal of fails despite adequate dosing during the first 12 weeks of treatment.
In line with other long-term follow-up studies, we found that severity of ADHD symptoms decreased significantly during the initial treatment period with regular and frequent clinical evaluations, but the beneficial effects decreased somewhat when the frequent follow-up by clinicians stopped. Overall, the study indicates that clinicians must assess and treat those who do not respond to ADHD medication during the first months of treatment with bigger efforts, and emphasize the importance of regular clinical monitoring and evaluation of the treatment strategy in childhood ADHD.
Availability of data and materials
The pseudonymous individual participant data that underlie the results reported in this article (text, tables, figures, and appendices) can be made available to investigators for individual participant data meta-analyses that have been approved by independent review committees. The data access will be granted on a case-by-case basis by the principal investigator (Tine Bodil Houmann) and the non-author point of contact, the data manager Michella Heinrichsen after further approval by the Capital Region of Denmark, Copenhagen, Denmark. Access will be granted to the extent permissible by the General Data Protection Regulation and the Danish Data Protection Act. Making the data available may require approval from the Danish Data Protection Authority. The pseudonymous data can be made available from 6 months after the publication date of this Article, and with no end date. Proposals for use of data and requests for access should be directed to tine.houmann@regionh.dk or michella.heinrichsen@regionh.dk. To gain access, researchers will need to sign a data access agreement with the Research Unit of the Child and Adolescent Mental Health Centre—Capital Region of Denmark, Copenhagen, Denmark. Software application: IBM SPSS Statistics version 25.0. Armonk, NY, USA: IBM Corp. 2017.
References
National Institute for Health and Care Excellence (2019) Guidelines. In: Attention deficit hyperactivity disorder: diagnosis and management. National Institute for Health and Care Excellence (NICE), London
Arnold LE, Hodgkins P, Caci H, Kahle J, Young S (2015) Effect of treatment modality on long-term outcomes in attention-deficit/hyperactivity disorder: a systematic review. PLoS ONE 10:e0116407
Barkley RAGE, Arthur LR (1999) Defiant teens. A clinician’s manual for assessment and family intervention. The Guilford Press, New York
Barkley RA, McMurray MB, Edelbrock CS, Robbins K (1990) Side effects of methylphenidate in children with attention deficit hyperactivity disorder: a systemic, placebo-controlled evaluation. Pediatrics 86:184–192
Bjerre D, Rasmussen HB (2017) Novel procedure with improved resolution and specificity for amplification and differentiation of variants of the gene encoding carboxylesterase 1. Pharmacogenet Genomics 27:155–158
Brinkman WB, Sucharew H, Majcher JH, Epstein JN (2018) Predictors of medication continuity in children with ADHD. Pediatrics. https://doi.org/10.1542/peds.2017-2580
Buitelaar JK, Van der Gaag RJ, Swaab-Barneveld H, Kuiper M (1995) Prediction of clinical response to methylphenidate in children with attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 34:1025–1032
Caye A, Spadini AV, Karam RG, Grevet EH, Rovaris DL, Bau CH, Rohde LA, Kieling C (2016) Predictors of persistence of ADHD into adulthood: a systematic review of the literature and meta-analysis. Eur Child Adolesc Psychiatry 25:1151–1159
Chen Q, Sjölander A, Runeson B, D’Onofrio BM, Lichtenstein P, Larsson H (2014) Drug treatment for attention-deficit/hyperactivity disorder and suicidal behaviour: register based study. BMJ 348:g3769
Cheung CH, Rijdijk F, McLoughlin G, Faraone SV, Asherson P, Kuntsi J (2015) Childhood predictors of adolescent and young adult outcome in ADHD. J Psychiatr Res 62:92–100
Cortese S, Adamo N, Del Giovane C, Mohr-Jensen C, Hayes AJ, Carucci S, Atkinson LZ, Tessari L, Banaschewski T, Coghill D, Hollis C, Simonoff E, Zuddas A, Barbui C, Purgato M, Steinhausen HC, Shokraneh F, Xia J, Cipriani A (2018) Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry 5:727–738
Dalsgaard S, Leckman JF, Mortensen PB, Nielsen HS, Simonsen M (2015) Effect of drugs on the risk of injuries in children with attention deficit hyperactivity disorder: a prospective cohort study. Lancet Psychiatry 2:702–709
DuPaul GJPT, Anastopoulos A, Reid R (1998) ADHD rating scale-IV. The Guilford Press, New York
Harold GT, Leve LD, Barrett D, Elam K, Neiderhiser JM, Natsuaki MN, Shaw DS, Reiss D, Thapar A (2013) Biological and rearing mother influences on child ADHD symptoms: revisiting the developmental interface between nature and nurture. J Child Psychol Psychiatry 54:1038–1046
Haugan AJ, Sund AM, Thomsen PH, Lydersen S, Nøvik TS (2021) Psychometric properties of the Weiss Functional Impairment Rating Scale parent and self-reports in a Norwegian clinical sample of adolescents treated for ADHD. Nord J Psychiatry 75:63–72
Kamimura-Nishimura KI, Brinkman WB, Froehlich TE (2019) Strategies for improving ADHD medication adherence. Curr Psychiatr 18:25–38
Keilow M, Holm A, Fallesen P (2018) Medical treatment of attention deficit/hyperactivity disorder (ADHD) and children’s academic performance. PLoS ONE 13:e0207905
Kvist AP, Nielsen HS, Simonsen M (2013) The importance of children’s ADHD for parents’ relationship stability and labor supply. Soc Sci Med 88:30–38
Kaalund-Brok K, Houmann TB, Hebsgaard MB, Lauritsen M-BG, Lundstrøm LH, Grønning H, Darling L, Reinert-Petersen S, Petersen MA, Jepsen JRM, Pagsberg AK, Plessen KJ, Rasmussen HB, Jeppesen P (2021) Outcomes of a 12-week ecologically valid observational study of first treatment with methylphenidate in a representative clinical sample of drug naïve children with ADHD. PLoS ONE 16:e0253727
Lahey BB, Lee SS, Sibley MH, Applegate B, Molina BSG, Pelham WE (2016) Predictors of adolescent outcomes among 4-6-year-old children with attention-deficit/hyperactivity disorder. J Abnorm Psychol 125:168–181
Lee SH, Song DH, Kim BN, Joung YS, Ha EH, Cheon KA, Shin YJ, Yoo HJ, Shin DW (2009) Variability of response time as a predictor of methylphenidate treatment response in korean children with attention deficit hyperactivity disorder. Yonsei Med J 50:650–655
Lichtenstein P, Halldner L, Zetterqvist J, Sjölander A, Serlachius E, Fazel S, Långström N, Larsson H (2012) Medication for attention deficit-hyperactivity disorder and criminality. N Engl J Med 367:2006–2014
McQuade JD, Breslend NL, Groff D (2018) Experiences of physical and relational victimization in children with ADHD: the role of social problems and aggression. Aggressive Behav 44:416–425
Molina BSG, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, Epstein JN, Hoza B, Hechtman L, Abikoff HB, Elliott GR, Greenhill LL, Newcorn JH, Wells KC, Wigal T, Gibbons RD, Hur K, Houck PR (2009) The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry 48:484–500
Mrug S, Molina BS, Hoza B, Gerdes AC, Hinshaw SP, Hechtman L, Arnold LE (2012) Peer rejection and friendships in children with attention-deficit/hyperactivity disorder: contributions to long-term outcomes. J Abnorm Child Psychol 40:1013–1026
Park S, Kim BN, Cho SC, Kim JW, Shin MS, Yoo HJ, Han DH, Cheong JH (2013) Baseline severity of parent-perceived inattentiveness is predictive of the difference between subjective and objective methylphenidate responses in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 23:410–414
Quinn PD, Chang Z, Hur K, Gibbons RD, Lahey BB, Rickert ME, Sjölander A, Lichtenstein P, Larsson H, D’Onofrio BM (2017) ADHD medication and substance-related problems. Am J Psychiatry 174:877–885
Roy A, Hechtman L, Arnold LE, Swanson JM, Molina BSG, Sibley MH, Howard AL (2017) Childhood predictors of adult functional outcomes in the multimodal treatment study of attention-deficit/hyperactivity disorder (MTA). J Am Acad Child Adolesc Psychiatry 56:687-695.e687
Ruiz-Goikoetxea M, Cortese S, Aznarez-Sanado M, Magallón S, Alvarez Zallo N, Luis EO, de Castro-Manglano P, Soutullo C, Arrondo G (2018) Risk of unintentional injuries in children and adolescents with ADHD and the impact of ADHD medications: a systematic review and meta-analysis. Neurosci Biobehav Rev 84:63–71
Schweren L, Hoekstra P, van Lieshout M, Oosterlaan J, Lambregts-Rommelse N, Buitelaar J, Franke B, Hartman C (2019) Long-term effects of stimulant treatment on ADHD symptoms, social-emotional functioning, and cognition. Psychol Med 49:217–223
Swanson JM, Arnold LE, Molina BSG, Sibley MH, Hechtman LT, Hinshaw SP, Abikoff HB, Stehli A, Owens EB, Mitchell JT, Nichols Q, Howard A, Greenhill LL, Hoza B, Newcorn JH, Jensen PS, Vitiello B, Wigal T, Epstein JN, Tamm L, Lakes KD, Waxmonsky J, Lerner M, Etcovitch J, Murray DW, Muenke M, Acosta MT, Arcos-Burgos M, Pelham WE, Kraemer HC (2017) Young adult outcomes in the follow-up of the multimodal treatment study of attention-deficit/hyperactivity disorder: symptom persistence, source discrepancy, and height suppression. J Child Psychol Psychiatry 58:663–678
Szomlaiski N, Dyrborg J, Rasmussen H, Schumann T, Koch SV, Bilenberg N (2009) Validity and clinical feasibility of the ADHD rating scale (ADHD-RS) A Danish Nationwide Multicenter Study. Acta Paediatr 98:397–402
van Lieshout M, Luman M, Twisk JW, van Ewijk H, Groenman AP, Thissen AJ, Faraone SV, Heslenfeld DJ, Hartman CA, Hoekstra PJ, Franke B, Buitelaar JK, Rommelse NN, Oosterlaan J (2016) A 6-year follow-up of a large European cohort of children with attention-deficit/hyperactivity disorder-combined subtype: outcomes in late adolescence and young adulthood. Eur Child Adolesc Psychiatry 25:1007–1017
Weiss M (2005) Weiss functional impairment rating scale—parent report (WFIRS-P). A new measure of impairment associated with ADHD. In: 158th Annual Meeting of the American psychiatric Association, Atlanta, GA
Weiss MD, McBride NM, Craig S, Jensen P (2018) Conceptual review of measuring functional impairment: findings from the Weiss Functional Impairment Rating Scale. Evid Based Ment Health 21:155–164
Wilk MBG, R. (1968) Probability plotting methods for the analysis of data. Biometrika 55:1–17
Zhang S, Faries DE, Vowles M, Michelson D (2005) ADHD Rating Scale IV: psychometric properties from a multinational study as a clinician-administered instrument. Int J Methods Psychiatr Res 14:186–201
Acknowledgements
We wish to thank all participating children and their families, and the staff and management from Child and Adolescent Mental Health Center, Mental Health Services–Capital Region of Denmark, Copenhagen, Denmark.
List of all partners in the INDICES Consortium. Lead author: Henrik Berg Rasmussen, henrik.berg.rasmussen@regionh.dk, Institute of Biological Psychiatry, Mental Health Centre Sct. Hans, Roskilde, Denmark. Ditte Bjerre, Institute of Biological Psychiatry, Mental Health Centre Sct. Hans, Copenhagen University Hospital, Roskilde, Denmark. Majbritt Busk Madsen, Institute of Biological Psychiatry, Mental Health Centre Sct. Hans, Copenhagen University Hospital, Roskilde, Denmark. Laura Ferrero, Institute of Biological Psychiatry, Mental Health Centre Sct. Hans, Copenhagen University Hospital, Roskilde, Denmark. Kristian Linnet, Section of Forensic Chemistry, Department of Forensic Medicine, Faculty of Health Sciences, University of Copenhagen, Denmark. Ragnar Thomsen, Section of Forensic Chemistry, Department of Forensic Medicine, Faculty of Health Sciences, University of Copenhagen, Denmark. Gesche Jürgens, Roskilde University Hospital, Unit of Clinical Pharmacology, Denmark. Kim Dalhoff, Department of Clinical Pharmacology, Bispebjerg University Hospital, Copenhagen, Denmark. Claus Stage, Department of Clinical Pharmacology, Bispebjerg University Hospital, Copenhagen, Denmark. Hreinn Stefansson, CNS Division, deCODE Genetics, Reykjavik, Iceland. Thomas Hankemeier, The Leiden/Amsterdam Center for Drug Research LACDR, Leiden University, Gorlaeus laboratories, Leiden, The Netherlands. Rima Kaddurah-Daouk, Department of Psychiatry and Behavioral Sciences, Duke University, Durham, NC, USA. Søren Brunak, Disease Systems Biology, Novo Nordisk Foundation Center for Protein Research, University of Copenhagen, Copenhagen, Denmark. Olivier Taboureau, Department of Systems Biology, Center for Biological Sequence Analysis, Technical University of Denmark, Lyngby, Denmark and INSERM, UMRS 973, MTi, Université Paris Diderot, Paris, France and Center for Biological Sequence Analysis, Technical University of Denmark, Kgs. Lyngby, Denmark. Grace Shema Nzabonimpa, Center for Biological Sequence Analysis, Technical University of Denmark, Kgs. Lyngby, Denmark. Tine Houmann, Centre for Child and Adolescent Mental Health, Research Unit, Mental Health Services, The Capital Region, Hellerup, Denmark. Pia Jeppesen, Centre for Child and Adolescent Mental Health, Research Unit, Mental Health Services, The Capital Region, Hellerup, Denmark Kristine Kaalund-Brok, Centre for Child and Adolescent Mental Health, Research Unit, Mental Health Services, The Capital Region, Hellerup, Denmark Peter Riis Hansen, Department of Cardiology, Copenhagen University Hospital, Hellerup, Denmark. Karl Emil Kristensen, Department of Cardiology, Copenhagen University Hospital, Hellerup, Denmark. Anne Katrine Pagsberg, Centre for Child and Adolescent Mental Health, Research Unit, Mental Health Services, The Capital Region, Hellerup, Denmark. Kerstin Plessen, Centre for Child and Adolescent Mental Health, Research Unit, Mental Health Services, The Capital Region, Hellerup, Denmark. Poul-Erik Hansen, Department of Science, Systems and Models, Roskilde University, Roskilde, Denmark. Wei Zhang, Department of Science, Systems and Models, Roskilde University, Roskilde, Denmark. Thomas Werge, Institute of Biological Psychiatry, Mental Health Centre Sct. Hans, Roskilde, Denmark.
Funding
This study was funded by Centre for Child and Adolescent Mental Health, Mental Health Services, The Capital Region of Denmark, Mental Health Services Research Fund, The Capital Region of Denmark, Fonden af 1982 J. nr. 6/17, The Beatrice Surovell Haskel Fund for Child Mental Health Research of Copenhagen J. nr. 11531, ID 359, Rosalie Petersens Fond J. nr. 020432-0001.
Author information
Authors and Affiliations
Consortia
Contributions
TBH, KJP, FV, NB, and PJ contributed to conception and design of the study. The data were collected and managed by TBH and KK-B. The data analyses were planned by TBH, MAP, NB, FV, and PJ and performed by TBH, KK-B, and LC. The first draft of the manuscript was written by TBH. NB, FV, and PJ commented on all previous versions of the manuscript. All authors read and approved the final version of the manuscript. The funding was organized by TBH.
Corresponding author
Ethics declarations
Conflict of interest
Tine Bodil Houmann has received speaker honorarium and travel support from Medice Nordic Denmark Aps. Kristine Kaalund Brok, Lars Clemmensen, Morten Aagaard Petersen, Kerstin Jessica Plessen, Niels Bilenberg, Frank Verhulst, and Pia Jeppesen all declare that they have no competing interests.
Ethics approval
The study was approved by the Danish Data Protection Agency (P-2019-851). The Local Committee on Health Research Ethics was consulted (J.nr. H-B-2009-026) in accordance with national guidelines and the 1964 Declaration of Helsinki. The study was evaluated not to be within their jurisdiction due to study design as an observational study. Participation was voluntary and data were kept confidential. The participants could withdraw their consent at any time without having to give reasons and with no consequences for their further treatment options.
Consent to participate
Informed consent was obtained from the children’s parents.
Consent for publication
All the authors have approved the manuscript and gave consent to submit.
Additional information
Membership of the INDICES Consortium is provided in the Acknowledgement.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Houmann, T.B., Kaalund-Brok, K., Clemmensen, L. et al. Early treatment response as predictor of long-term outcome in a clinical cohort of children with ADHD. Eur Child Adolesc Psychiatry 33, 357–367 (2024). https://doi.org/10.1007/s00787-023-02158-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00787-023-02158-z