Abstract
Transcranial direct current stimulation (tDCS) has demonstrated benefits in adults with various psychiatric disorders, but its clinical utility in children and young people (CYP) remains unclear. This PRISMA systematic review used published and ongoing studies to examine the effects of tDCS on disorder-specific symptoms, mood and neurocognition in CYP with psychiatric disorders. We searched Medline via PubMed, Embase, PsychINFO via OVID, and Clinicaltrials.gov up to December 2022. Eligible studies involved multiple session (i.e., treatment) tDCS in CYP (≤ 25 years old) with psychiatric disorders. Two independent raters assessed the eligibility of studies and extracted data using a custom-built form. Of 33 eligible studies (participant N = 517), the majority (n = 27) reported an improvement in at least one outcome measure of disorder-specific symptoms. Few studies (n = 13) examined tDCS effects on mood and/or neurocognition, but findings were mainly positive. Overall, tDCS was well tolerated with minimal side effects. Of 11 eligible ongoing studies, many are sham-controlled RCTs (n = 9) with better blinding techniques and a larger estimated participant enrolment (M = 79.7; range 15–172) than published studies. Although encouraging, the evidence to date is insufficient to firmly conclude that tDCS can improve clinical symptoms, mood, or cognition in CYP with psychiatric disorders. Ongoing studies appear of improved methodological quality; however, future studies should broaden outcome measures to more comprehensively assess the effects of tDCS and develop dosage guidance (i.e., treatment regimens).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is estimated that 10–20% of children and adolescents experience mental health disorders worldwide [1], with roughly 75% of all psychiatric disorders having an onset in childhood, adolescence, or early adulthood (mid-20 s) [2, 3]. This period of onset coincides with sensitive periods of experience-dependent changes in brain structure and function, with evidence showing common and disorder-specific functional disorganisation of neurocognitive and affective networks in children and young people (CYP) with psychiatric disorders (e.g. [4].). This highlights the need for early detection and intervention [5]; however, pharmacotherapy in CYP remains contentious across many mental health conditions [6, 7] and the widespread imbalance between demand and capacity in child and adolescent mental health services may limit CYP from accessing timely, quality mental health care [8].
Neuromodulation techniques, particularly non-invasive brain stimulation, are safe and promising treatment alternatives and/or adjuncts that could be used to bridge the mental health treatment gap [9]. One particular technique, transcranial direct current stimulation (tDCS), involves the application of low amplitude (e.g., 1–2 mA), sustained current over a short duration (e.g., 20 min) via strategically positioned electrodes on the scalp [10]. From a mechanistic perspective, the short- and longer-term effects of tDCS on cortical excitability are polarity-specific, i.e., anodal tDCS increases excitability of local neurons and cathodal TDCS decreases excitability [11]. The immediate effects of tDCS relate to a shift in resting transmembrane potential of the neurons stimulated [12], whereas post-stimulation effects are proposed to rely on N-methyl-D-aspartate (NMDA) glutamate receptor-dependent neuroplastic changes, similar to those occurring in long-term potentiation (LTP) and depression (LTD) [13]. Evidence has also shown that excitatory after-effects of anodal tDCS are mediated by a reduction in intracortical gamma aminobutyric acid (GABA), whereas cathodal tDCS after-effects are mediated by reduced glutamate concentrations [14,15,16]. Modulation of neural activity in regions under the stimulating electrode [17], as well as in distal, interconnected regions [18], makes tDCS ideal for use in neuropsychiatric disorders associated with hyper- or hypo- cerebral excitability patterns.
The duration and strength of tDCS after-effects on neuronal excitability and/or synaptic strength is contingent upon the stimulation parameters used (e.g., intensity, duration, location, and number of sessions) (e.g., [19]), as is its safety and tolerability. A comprehensive review [20] reported no serious/irreversible adverse events in over > 33,000 sessions of tDCS applied at < 4 mA, for < 40 min, and a total charge of < 7.2 Coulombs. A recent systematic review [21] of safety and tolerability in 156 CYP found that 864 tDCS sessions applied within the standards (i.e., 0–2 mA, 10–20 min, 1–20 sessions) produced no serious/irreversible adverse events and reported side-effects were similar to those in adults, with tingling (25–58%) and itching (25–54%) the most frequently reported. This, in combination with its portability and relatively low cost, makes tDCS a promising therapeutic tool for CYP with psychiatric disorders.
In psychiatric research, tDCS has been mainly administered to adults. The data have resulted in meta-analytic evidence and promising reviews of beneficial effects (e.g. [22, 23]). Studies involving CYP are growing rapidly, with > 20 studies published or registered as trials on clinicaltrials.gov in the past 2-years (see also, [24, 25]). Despite this, attempts to consolidate findings in CYP are outdated or limited to non-systematic, narrative reviews [26, 27] or systematic reviews that have focused on literature for a specific disorder, particularly neurodevelopmental disorders [28, 29], or the safety and tolerability of tDCS [21]. None have examined tDCS-effects on mood or cognition in CYP with psychiatric disorders. Therefore, we followed a rigorous methodology for systematic reviews focusing on published and unpublished studies in CYP with psychiatric disorders.
Following PRISMA 2020 (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [30], we systematically reviewed studies investigating tDCS effects across psychiatric disorders in CYP in order to (1) evaluate the effects of tDCS on disorder-specific symptoms and impairments, (2) determine the effects of tDCS on mood and neurocognitive outcomes, and (3) outline the populations and methodologies used in ongoing trials and unpublished data.
Methods
Protocol and registration
This study was pre-registered (see PROSPERO, ID: CRD42019158957; and [31]) and is reported in line with PRISMA 2020 guidelines [30].
Literature search
MEDLINE, EMBASE and PsycINFO databases were searched using the following search terms: (transcranial direct current stimulation or tDCS) AND (young people, child, adolescent, young adult, youth, boy, girl, paediatric, young people and young persons) AND (neuropsychiatric disorders, autism, ADHD, schizophrenia, mood disorder, bipolar, depression, anxiety, panic, OCD, Tourette’s, PTSD, acute stress disorder, substance abuse and eating disorders, personality disorder). The search was conducted on 28/01/21 and was updated on 26/01/22 and 09/12/22. The reference lists of included studies were manually searched for additional relevant studies not identified by the database search. To identify ongoing/unpublished trials, we searched World Health Organisation International Clinical Trials Registry Platform (ICTRP) registry, the National Institute of Health (NIH) registry, the European Union Clinical Trials Register, and the International Standard Randomised Controlled Trials Number (ISRCTN) registry.
Eligibility criteria
We included all types of full-text publications written in English that reported multiple (> 1) sessions of tDCS in individuals under 26 years of age at enrolment with a psychiatric disorder. We included all types of reports, studies and multi-session tDCS protocols unless the aim was basic research, protocol development, or to investigate the mechanism of action of tDCS.
Data extraction and analysis
Two authors (LG and YL) independently screened identified records against the eligibility criteria, extracted data, and performed the quality assessment. Data extraction was performed with a custom-made form adapted from the Cochrane data collection for intervention reviews [32] (see Supplementary Material S1 for details). A meta-analysis was not feasible due to significant heterogeneity in study designs, outcome measures, and tDCS protocols.
Quality assessment
LG and YL independently assessed risk of bias using the Cochrane risk of bias 2.0 tool (RoB 2.0) in randomised controlled trials (RCTs) [33] and the Cochrane tool for risk of bias in non-randomised studies of interventions (ROBINS-I) [34]. Inter-rater agreement was 92%. Conflicts were resolved by discussion.
Results
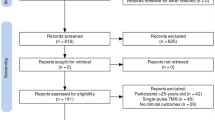
We identified 33 eligible studies (total N = 517; age range 2–25 years, M = 15.53, SD = 6.44; 82.2% male), composed of eight double-blind RCTs [37,38,39,40, 43, 44, 46, 48], four double-blind, crossover RCTs [35, 43, 45, 47], one double-blind, sham-controlled trial [67], two single-blind RCTs [41, 63], one single-blind controlled clinical trial [65], four open-label studies [36, 38, 44, 62], and 14 case series/studies [42, 51,52,53,54,55,56,57,58,59,60,61, 64, 66] (see Table 1 and 2). Seven studies were in ASD [35,36,37,38,39,40,41,42,43], seven in ADHD [44,45,46,47,48,49,50], four in schizophrenia [51,52,53,54], two in OCD [55, 56], Tourette’s syndrome [57, 58], depression [59, 60], anxiety [61, 62], or substance abuse disorder [63, 64], and one each in eating disorders [65], catatonia [66], or co-comorbid ASD, ADHD and anxiety [67]. Across studies, tDCS was typically delivered for 20 to 30 min (M = 21.61; SD = 4.38) once-daily over 4 to 28 sessions (M = 10.75; SD = 6.15). However, several studies applied tDCS in twice-daily sessions [51, 53,54,55,56,57, 67], one of which suggested that tDCS remains an ongoing treatment [51]. Montage configurations included anodal (n = 15), cathodal (n = 3), and bilateral (n = 15) tDCS, with anodal tDCS to the left-DLPFC (n = 10) and bilateral tDCS to the DLPFC (n = 6) the most frequently used across studies. tDCS was delivered at a stimulation intensity between 0.25 and 3 mA, with 1 mA (n = 13) or 2 mA (n = 11) most employed (Fig. 1).
Quality assessment
Of the 14 RCTs, overall risk-of-bias was rated as “high” in eight studies [39,40,41, 43, 45, 48, 63, 67], and six with “some concerns” [35, 37, 46, 47, 49, 50] (see Supplementary Material S2). The non-randomised, controlled clinical trial was rated with moderate risk of bias [65]. All open-label studies, case series, and case reports were rated as low quality.
Four RCTs were prospectively registered [40, 45, 48, 50], and three were retrospectively registered [43, 47, 63]. Among these seven protocols or trial registries, we found inconsistent and/or selective reporting in four studies. For example, one trial registered actual enrolment (i.e., updated following study completion) of 105 participants, inclusion of a wait-list control group, and a 6-month follow-up timepoint. In the final publication [40], only 41 participants were randomly assigned to receive real or sham tDCS (i.e., no wait-list control group) and there was no follow-up period. In addition, four registered primary outcomes (Early Adolescent Temperament Questionnaire; Autism Quotient (AQ); N-Back Task; and Attention Network Task) were omitted from the final publication [40], and the Cambridge Neuropsychological Test Automated Battery (CANTAB) was partially reported and relegated from a registered primary outcome to a secondary outcome. Two other studies omitted registered primary outcome measures (AQ and Gresham & Elliot Social Skills Rating Scale [43]; Dot-Probe Task and Difficulties in Emotion Regulation Scale [63]) and/or secondary outcome measures (Executive Function Checklist [43]) from final publication. Another study omitted a registered secondary outcome (Behaviour Rating Inventory of Executive Function), partially reported the Wechsler Intelligence Scale for Children, and promoted an outcome registered as “other” to a secondary outcome in the final publication (Clinical Global Impression—Severity [45]). Finally, one RCT [37] reported an incorrect clinicaltrials.gov identifier (NCT number) and we were unable to find the study record using other trial information (e.g., investigator name) in the advanced search function.
Clinical tDCS effects in psychiatric disorders
Neurodevelopmental Disorders
Autism spectrum disorder (ASD)
Five studies applied anodal tDCS to the DLPFC in CYP with ASD. A recent multi-arm RCT [37] assigned 36 children with ASD to receive either (a) 20 sessions of anodal-tDCS to the left-DLPFC, (b) 5 sessions of anodal-tDCS to the left-DLPFC followed by 15 sessions of sham-tDCS, or (c) 20 sessions of sham-tDCS. Compared to sham, 5- and 20-sessions of anodal-tDCS were associated with significant improvements in clinician-rated total ASD severity and related symptoms, including physical health and behaviour, language, and sociability, but not sensory and cognitive awareness, at day-5, day-14, and at 6-month follow-up [37]. At 12-month follow-up, only improvements in clinician-rated total ASD severity and sociability remained significant in the 20-session tDCS group (vs. sham). Of note, no significant differences in total ASD severity or related behaviours were detected between the 5- and 20-session tDCS groups at any time point [37].
Another sham-controlled RCT [39] in 43 children with ASD reported a significant reduction in clinician-rated total ASD severity and related symptoms, including sociability, physical health and behaviour, but not sensory and cognitive awareness or language, immediately after 10-sessions of bilateral tDCS to the DLPFC (anode: left; anode: right) compared to sham. A recent single-blind RCT [41] in 40 children with ASD reported a significant improvement in sleep and observer-rated overall ASD severity immediately after 15-sessions of anodal tDCS over the left-DLPFC compared to sham, while ASD symptoms and related behaviour impairments remained unchanged [41].
The remaining two studies applied 5 sessions of anodal tDCS over the left-DLPFC: one crossover RCT in 20 children with ASD [35] reported a significant improvement in investigator- and parent-ratings of ASD symptoms, as well as parent-rated sociability, physical health and behaviour, sensory and cognitive awareness compared to sham, but not speech and language communication, at 1-week post-stimulation. Ratings of psychosocial functioning also improved at 1-week post-stimulation compared to sham, while clinical impression of improvement was rated as “much improved” in nine and “minimally improved” in eight children [35]. A single-arm open-label study [36] in 10 children with ASD reported a significant reduction in investigator-rated ASD symptoms immediately, 1 week, and 2 weeks post-treatment, compared to baseline, whereas parent-ratings of ASD symptoms relating to sociability, physical health and behaviour, sensory and cognitive awareness, but not speech and language communication, were significantly reduced at post-treatment only [36].
Only one study applied bilateral tDCS to the DLPFC (anode-left; cathode-right) in 32 children with ASD. In this double-blind RCT [43], the tDCS group showed significant improvements in (a) parent-rated emotion regulation competencies, (b) clinician-rated stereotyped behaviour, and (c) theory of mind (ToM), including first- and second-order beliefs, immediately and 1 month after 10 sessions of 1.5 mA bilateral tDCS, compared to sham. However, no significant between-group differences were detected in clinician-rated overall ASD severity and symptoms relating to communication, sociability, behavioural difficulties and precursors of ToM [43].
Two studies applied cathodal tDCS to CYP with ASD: a recent double-blind, sham-controlled RCT [40] in 41 CYP with ASD reported a significant group by time interaction in parent-rated severity of social deficits and restricted interests and repetitive behaviours due to improvement from baseline to immediately after 10 sessions of 1.5 mA tDCS to the left-DLPFC with concurrent cognitive remediation training (CRT), but not sham tDCS + CRT. A single-arm open-label study [38] applied 20 sessions of cathodal tDCS over the right cerebellar lobe in seven children with ASD, which reduced caregiver-rated aberrant behaviour symptoms 1 week after stimulation compared to baseline in all but two participants, both of whom were taking psychotropic medication during the study period. Unexpectedly, 1 week after stimulation, one patient with a history of epilepsy showed no EEG-related epileptic activity in the frontal region, while another participant with comorbid tic disorder showed fewer, less intense tics, which remained until 3-month follow-up [38].
Last, a case report [42] in an 18-year-old male with ASD applied 8 sessions of anodal tDCS to the right temporoparietal junction and reported fewer total ASD symptoms immediately and 2-months after stimulation compared to baseline.
Attention deficit hyperactivity disorder (ADHD)
Two double-blind RCTs applied tDCS to the inferior frontal cortex (IFC). The largest RCT [50] in CYP with ADHD (n = 50) administered 15 sessions of anodal or sham tDCS to the right-IFC with concurrent cognitive training (CT). Compared to sham, the tDCS group showed significantly higher parent-rated ADHD symptoms immediately after stimulation, but not at a 6 months’ follow-up. No significant differences were reported in other measures of ADHD symptoms or related impairments [50]. The other RCT [46] administered five sessions of bilateral high-definition tDCS to the right- and left-IFC in 33 children and adolescents with ADHD, with stimulation intensity titrated post-randomisation to 0.25 mA (n = 11) and 0.5 mA (n = 9) to minimise discomfort. Results showed a significant reduction in self-rated ADHD total and hyperactivity symptoms compared to sham at post-treatment, but not at 4-month follow-up, while self-rated inattention or impulsivity and all parent-rated ADHD symptoms remained unchanged [46].
Four studies applied anodal tDCS to the left-DLPFC: a recent double-blind, sham-controlled RCT [48] with 25 CYP with ADHD reported no significant between-group differences in parent-rated severity of ADHD symptoms or related behaviours immediately after 12 sessions of 1 mA tDCS with concurrent CT, compared to sham tDCS + CT. A smaller double-blind, crossover RCT [49] in 15 adolescents with ADHD reported significant improvement in parent-rated inattention at 1-week follow-up, but not immediately after five sessions of tDCS, compared to sham. No other clinical effects were found [49]. Another double-blind, crossover RCT [45] in 19 children with ADHD compared five sessions of transcranial random noise stimulation (tRNS) over the left-DLPFC and right-IFC with tDCS to the left-DLPFC, both with concurrent executive function training. Findings showed that relative to tDCS, tRNS significantly reduced parent-rated ADHD symptoms immediately and 1 week after stimulation (adjusting for baseline scores), but with no difference in global clinical impressions [45].
Last, in a single-arm open-label study [44] in nine children with ADHD, five sessions of anodal tDCS over the left-DLPFC were combined with a picture association cognitive training task and parents reported overall improvements in behaviour. However, without a sham-control, a placebo effect cannot be ruled out.
ASD and ADHD
A double-blind, parallel, sham-controlled case report [67] applied 10-sessions of anodal-tDCS over the right inferior frontal gyrus (rIFG) combined with cognitive training in two 15-year-old, female, fraternal adolescent twins with ASD, ADHD, and anxiety. Both twins had parent-reported compulsive symptoms, and one had comorbid OCD. Compared to baseline, findings showed reduced parent-rated compulsive and repetitive/restrictive symptoms, but not clinician-rated OCD symptoms or parent-rated ADHD symptoms, immediately after anodal-tDCS. No clinical changes were observed following sham-tDCS [67].
Tourette’s syndrome
In one case study [57] in an 18-year-old female and 20-year-old male with motor and vocal tics, bilateral cathodal tDCS was applied to the right- and left-pre-supplementary motor area (SMA) twice-daily over 5 consecutive days. Compared to baseline, one participant showed a reduction in tics immediately after stimulation, whilst the other participant showed an increase in tics and OCD symptoms. Both participants’ self-rated negative affect decreased, and positive affect increased, but none of these changes were statistically tested [57]. One case study in a 16-year-old boy with refractory Tourette’s syndrome showed reduced motor and vocal tics immediately, 3-, and 6-weeks after ten sessions of cathodal tDCS over the left pre-SMA compared to baseline [58].
Schizophrenia-spectrum disorders
Schizophrenia
Three case studies applied bilateral tDCS (anode-left-DLPFC; cathode-right-temporo-parietal junction) over 5 consecutive days. In two of these studies, twice-daily tDCS reduced auditory hallucinations entirely at post-treatment in a 24-year-old male [53] and almost entirely 1-month after stimulation in a 25-year-old pregnant female [54], whereas in the third study [51], once-daily tDCS reduced auditory hallucinations immediately after stimulation in a 25-year-old female, but she required continued once- or twice-daily stimulation to maintain improvements over 3 years. Last, one case study in a 19-year-old-male reported reduced positive and negative symptoms, disorganization, flattened affect, lack of concentration, and impetus after ten sessions of anodal-tDCS over the left-DLPFC [52].
Catatonia
A case study [66] in a 14-year-old female with ASD and catatonia showed reduced catatonic symptoms compared to baseline immediately and 1-month after 28-sessions of bilateral tDCS (anode-right; cathode-left) over the DLPFC.
Major depressive disorder
Two case studies applied ten sessions of bilateral tDCS over the left- and right-DLPFC. One study [59] reported that a 21-year-old male presenting with a moderate depressive episode showed fewer depressive symptoms immediately after 5-sessions of stimulation applied on two occasions separated by 2 years. The second [60] reported that a 23-year-old pregnant female with a depressive episode showed fewer depressive and anxiety symptoms 1 month after stimulation compared to baseline, which, the authors suggested was indicative of clinical remission.
Anxiety disorders
A single-arm, open-label study [62] in six adolescents with social anxiety disorder or generalized anxiety disorder reported that combined anodal-tDCS to the left-DLPFC with attention bias modification training reduced self- and parent-reported total anxiety symptoms, self- and parent-rated anxiety-related emotional symptoms, and clinician-rated anxiety symptoms, compared to baseline. However, only self-reported total anxiety symptoms significantly reduced from baseline to post-stimulation [62].
A case study [61] in a 24-year-old female with social anxiety disorder reported reduced self-reported social anxiety symptoms immediately after five sessions of anodal tDCS over the left vmPFC, and at 15 days’ follow-up, although improvements did not reach clinical significance.
Obsessive compulsive disorder (OCD)
Two case studies applied anodal tDCS over the left pre-SMA/SMA twice-daily over 10 conseuctive days. In one [55], a 24-year-old male with OCD and co-occurring depressive symptoms showed fewer OCD symptoms compared to baseline immediately and 7 months after stimulation. The same stimulation protocol was applied for eight sessions 2 years later, which reduced recurring OCD and depressive symptoms, but also lesioned the skin under the stimulation site [55]. In the second case study [56], a 24-year-old male showed a reduction in OCD, depression, and anxiety symptoms immediately, 1 week and 1 month after stimulation.
Substance abuse disorder
A single-blind, parallel group RCT [63] in 80 boys with methamphetamine addiction administered 12-sessions of anodal tDCS over the left-DLPFC with or without combined mindfulness training, mindfulness training only and sham tDCS only. The results showed a significant reduction in desire for drugs at post-treatment and 1-month follow-up in the three active treatment groups, but not in the sham group, compared to baseline [63].
In a case report [64] in a 24-year-old male with methamphetamine use disorder, anodal-tDCS over the right DLPFC reduced self-reported drug cravings immediately after 20 sessions of stimulation and, after four more sessions, at 6 months’ follow-up, at which point paranoid delusions and hallucinations had also reduced completely.
Eating disorders
One clinically controlled trial [65] in 23 adolescents with anorexia nervosa (AN) combined treatment as usual with either family therapy or 18-sessions of bilateral tDCS over the DLPFC (anode-left; cathode-right). It was not clear how people were allocated to these two study arms. Compared to baseline, only the tDCS group had significantly reduced BMI immediately after stimulation and at 1-month follow-up. Both groups showed significantly reduced overall AN, depression, and anxiety symptoms compared to baseline, but no significant Group by Time interaction, and thus, placebo effects cannot be ruled out [65].
Mood tDCS effects in psychiatric disorders
ADHD
A double-blind, sham-controlled RCT [48] reported no significant differences in parent-rated mood or anxiety scores following 12 sessions of anodal-tDCS to the left-DLPFC with cognitive training (CT) compared to sham + CT.
OCD
Two case studies [55, 56] reported reduced clinician-rated depression symptoms on the Hamilton Depression Rating Scale following anodal-tDCS to the SMA compared to baseline.
Substance abuse disorder
In a case report [64], the participant self-reported reduced depression, on the Beck Depression Inventory immediately, 3-months, and 6-months after anodal-tDCS to the right-DLPFC compared to baseline.
Neurocognitive tDCS effects in psychiatric disorders
ASD
A double-blind RCT [40] reported that improvements in parent-reported social communication and reduced restricted, repetitive behaviours were significantly associated with improved emotion recognition (CANTAB Emotion Recognition Task) and cognitive flexibility (composite score of: (1) time taken to complete the Color Trail Test 2, (2) switch cost in the CANTAB Multitasking Test, and (3) mean reaction time during the WCST rule-switching block) following cathodal tDCS to the left-DLPFC + CRT, compared to sham + CRT. In addition, results showed significant improvements in information processing (composite score of: (1) time taken to complete the Color Trail Test 1, and (2) CANTAB Reaction Time Test) following cathodal-tDCS + CRT, compared to sham [40].
ADHD
A recent RCT [48] reported no significant between-group differences in neurocognitive performance on the CANTAB after 12-sessions of 1 mA tDCS to the left-DLPFC combined with cognitive training (CT), compared to sham tDCS + CT. Another recent sham-controlled, crossover study [47] in 11 CYP with ADHD reported a significant reduction in number of omission errors (i.e., inhibitory control) in the real tDCS group, compared to sham, immediately after receiving 5 sessions of 1.5 mA cathodal tDCS to the left-DLPFC, but not at 1-week or 1-month follow-up. No significant between-group differences were detected for auditory continuous performance (i.e., sustained attention) immediately, 1-week, or 1-month after real tDCS, compared to sham tDCS [47].
A double-blind RCT [46] comparing 0.5 mA and 0.25 mA to sham reported a significant reduction in reaction time variability in a combined Go/No-Go and n-back task immediately and 4-months after 5-sessions of 0.5 mA anodal HD-tDCS over the right IFC. In contrast, in the same task, the 0.25 mA group showed an increase in no-go commission errors over the course of tDCS, and this effect became significant at day-5, but was non-significant at post-stimulation and at 4-month follow-up. At post-stimulation, the 0.5 mA group also showed a significant reduction in reaction time variability in the flanker task and a reduced number of commission errors in the spanboard task compared to sham, but neither effect was significant at follow-up [46].
One double-blind, crossover trial [49] showed that compared to sham, anodal tDCS improved (a) QbTest (a combined working memory (n-back minus-2) and go/no-go task) measures of attention at 7-day follow-up only and, (b) measures of hyperactivity immediately and 7-days after stimulation, but not measures of impulsiveness. In a double-blind, crossover RCT [45], tRNS improved working memory, but not short-term memory, and only processing speed in a sustained attention task, compared to tDCS. In addition, exploratory moderation analysis predicted a trend-level larger tRNS effect in parent-rated ADHD symptoms for participants with the greatest working memory improvement.
In a double-blind, parallel RCT [50], there were no significant effects of anodal-tDCS to the right-IFC across measures of motor and interference inhibition, time estimation, sustained attention, cognitive flexibility, visuospatial working memory, and three task-independent measures processing speed, intrasubject response variability, and prematurity, compared to sham. Finally, a single-arm open-label study [44] reported a significant reduction in errors on attention (omission) and switch tasks after anodal-tDCS to the left-DLPFC compared to baseline, but no improvement in verbal or visuospatial working memory.
Schizophrenia
One case study [52] reported faster completion time on the Trail Making Test (TMT) Part A and B and fewer errors on the Self-Ordered Pointing Task (SOPT) one- and two-weeks after anodal-tDCS to the left-DLPFC compared to baseline.
Substance abuse disorder
A single-blind, parallel RCT [63] reported a significant group by time interaction in n-back task reaction times and accuracy, Wisconsin Card Sorting Task perseverative errors, and in a risk-taking task, all due to an improvement from baseline to immediately and 1-month after (a) tDCS only, (b) tDCS + mindfulness training, or (c) mindfulness training only, but not sham. However, there were no equivalent effects on any of the go/no-go task measures.
A case study [64] reported consistent improvement on subscales of the Cognitive Abilities Questionnaire that measured memory, inhibitory control, selective attention, decision making, planning, sustaining attention, and cognitive flexibility, but not social cognition, from baseline to 2-months, 4-months, and 6-months after anodal-tDCS to the right-DLPFC.
Safety
Overall, tDCS was well-tolerated and feasible in a variety of age groups and psychiatric disorders, which extends existing evidence of a good side-effect and tolerability profile of tDCS in children and adolescents [20]. However, adverse events (AEs) were not measured or reported in six studies [43, 47, 52, 56, 58, 66] and whilst four studies reported no AEs, it was not clear whether sensory side effects (e.g., tingling sensation) were measured [35, 36, 39, 54]. Only 10 studies reported monitoring AEs actively (i.e., using a structured questionnaire that lists specific AEs), whereas the remaining 16 studies monitored AEs passively and often relied on spontaneous feedback from participants or caregivers (see Table 1 & 2). Here, a selective reporting bias is very likely as the frequency of AEs reported increases when monitored actively [68]. Future studies should collect data for AEs actively, using a structured questionnaire (e.g., [68]) in which the rater asks for each specific AE (e.g., headache or itching).
Across studies, one severe adverse event (SAE) was reported, which was an erythematous lesion. The lesion was ~ 1 cm in diameter at the site of stimulation and developed during the third session of the patient’s second course of tDCS (patient had received 20 tDCS sessions 7-months prior) [49]. The authors noted that the lesion was not experienced as itchy or painful, and that it resolved spontaneously. Skin lesions and/or thermic damage appear to be rare and likely result from improper tDCS preparation or administration (e.g., poor electrode skin contact from dry sponges) [69, 70]. Therefore, it is imperative that the condition of tDCS electrodes is closely monitored over time and that care is taken when administering saline to the sponge of electrodes to prevent tDCS-related skin damage.
Unpublished registered trials
Of the 11 registered trials (see Table 3), four have not started recruiting, one has been completed (October 2021), and the remaining six are ongoing. Three are quadruple-blind RCTs, four are triple-blind (one crossover; three RCTs), two are double-blind RCTs, two are open label (one single-arm; one-RCT). These 11 studies are (a) recruiting either ASD (n = 6), ADHD (n = 2), or MDD (n = 3); (b) stimulating the DLPFC (n = 7), temporal parietal junction (n = 1), or using a neuroimaging biomarker (n = 1); (c) applying tDCS alone (n = 2), or combining stimulation with cognitive training (n = 4), mindful breathing training (n = 1), applied behaviour therapy (n = 1) or medication (n = 3; and (e) recruiting ~ 80 participants (range: 15–172) per trial, in CYP aged (on average) between 10 and 18 years old.
Discussion
This is the first systematic review that collates published and unpublished studies investigating the effects of multi-session tDCS applied to CYP with psychiatric disorders. To date, studies are limited to case studies/series (n = 14), open-label single-arm studies (n = 4), sham- or active-controlled trials with < 50 participants (n = 13) or > 50 participants (n = 2). These studies demonstrate tDCS is well-tolerated, and that it is feasible to conduct RCTs in CYP, particularly those with ADHD and ASD. There is some encouraging evidence of improvement in clinical, cognitive, or mood measures, however, it is not possible to determine the therapeutic efficacy of multi-session tDCS for CYP with psychiatric disorders.
Of the 33 included studies, 30 measured clinical effects immediately after the final tDCS session, with all except six [45, 46, 48,49,50, 57] reporting an improvement in at least one outcome measure of core disorder-specific symptoms. Of the 19 studies that measured clinical effects at a longer-term follow-up, improvements in core symptoms persisted at 1-week [36, 56], 2-weeks [36, 37, 61], 3-weeks [58], 1-month [43, 54, 56, 60, 63, 65, 66], 6-weeks [58] 6-months [37, 64], 7-months [55], or 12-months [37] after the final session of tDCS, with one study reporting no effect at 4-months [46]. Overall, these findings are in line with evidence of improvement in clinical outcomes in adults with psychiatric disorders, which have been shown to persist up to at least one-month post-stimulation (e.g. [29]). Interestingly, clinical effects only persisted on a once- to twice-daily tDCS maintenance regime in one case [51] or were significantly improved at 1-week but not immediately after stimulation [49]. This might relate to findings showing delayed tDCS effects, such that improvements only emerge after the acute treatment phase [73]. However, the evidence of carryover effects in Soff et al., [49] limited their analyses to the first phase of the crossover study with a very small sample size (N = 15). Therefore, larger parallel-group RCTs are needed to examine changes in core and related symptoms of psychiatric disorders in CYP immediately after tDCS as well as at one or multiple longer-term follow-ups.
Relatively few studies measured neurocognitive or mood outcomes. 11 studies measured neurocognitive outcomes, of which seven found tDCS-related improvements in the first assessment immediately [40, 44, 46, 47, 63], 1-week [52], or 1-month [64] after the last stimulation session, which persisted in five of the studies until 1-week [49], 2-weeks [52], 1-month [63], 4-months [46], and 6-months [64] follow-up. Three studies reported no effect of tDCS on cognition compared to sham [48, 50] or tRNS [45] at post-stimulation and/or follow-up. Three of the four studies measuring mood outcomes found improvements immediately after tDCS [55, 56, 64], with one study testing and finding the effect at 3- and 6-months follow-up [64]. One study reported no effect of tDCS on mood compared to sham [48].
In the 13 studies with cognitive and/or mood outcomes, all except four [46, 50, 55, 56] stimulated the DLPFC. This is in line with substantial meta-analytic evidence that the DLPFC subserves executive functions or regulates mood [e.g. [74,75,76,77].), and with tDCS studies in healthy controls, which have reported improved cognitive outcomes up to 12-months [78] and in mood outcomes [79] post-stimulation. Twelve other studies also stimulated the DLFPC, but none measured cognitive or mood outcomes. Impaired executive functioning and mood regulation mediate the pathophysiology of many psychiatric disorders (depression [80], schizophrenia [81], ADHD [82], and ASD [83]). Further, patients often desire alternative treatments that improve executive functioning or mood over symptoms [84] without side effects associated with pharmacological interventions, such as secondary blunted affect [85], weight gain and poor social functioning [86]. It is therefore important that future research measure the effects of tDCS on a variety of disorder-relevant cognitive outcomes and mood impairments.
Heterogeneous stimulation protocols and lack of dosage-guidance meant we were not able to identify optimal stimulation parameters in CYP with psychiatric disorders. This is of concern given the neurophysiological effects of tDCS may be non-linear, and because emerging evidence suggests tDCS may modulate cortical excitability via the scalp and/or peripheral nerves, which may complicate predictions about the dose–response relationship and the reproducibility of findings [87]. For example, in one RCT [46], adolescents with ADHD received sham (n = 13), or 0.5 mA (n = 9), or 0.25 mA (n = 11) anodal HD-tDCS to the IFC depending on cutaneous sensitivity. Compared to sham, the 0.25 mA group showed significantly reduced response inhibition, an effect not observed in the 0.5 mA group, which opposed the authors hypothesis that increasing right-IFC activity would improve executive function. In addition, in one case series [57], cathodal tDCS to the motor cortex did not improve symptoms in Tourette’s syndrome, and actually increased tic-count, whereas in another case report [58], cathodal tDCS to the pre-SMA improved both motor and verbal tics.
The majority of studies (n = 30) used a stimulation intensity of 1–2 mA, based on studies in adult populations. However, the stimulation intensity required to modulate cortical excitability in a polarity-dependent manner or induce longer-term effects that persist after stimulation cessation in CYP may differ from that in adults [88]. The issue of safety in tDCS in CYP is often regarded as not being a major concern given that both the type and the magnitude of adverse effects do not differ between CYP and adults. However, it is of note that various anatomical parameters change with age (e.g., scalp-to-brain distance) and evidence shows that in CYP, lower current intensities (e.g., 1 mA) can achieve brain current densities seen in adults at 2 mA current [88, 89]. Application of tDCS on the basis of parameters used in adults may, therefore, produce larger and potentially unintended or adverse effects in CYP. An example of unanticipated findings (albeit positive, rather than adverse) was reported in D’Urso et al.’s [38] study of cathodal tDCS applied to the cerebellum of CYP with ASD. It reported the remission of two frontal epileptic foci in one participant who had lifelong comorbid epilepsy and in another participant who had comorbid tic disorder, there was a 90% improvement in frequency and intensity of tics, and this was maintained until 3-month follow-up. This could be evidence of indirect stimulation of non-target sites [90], leading to possible unintended modulation of symptoms, behaviour or cognition in a potentially clinically meaningful manner. This is relevant in the wider ethical debate surrounding direct-to-consumer marketing of tDCS devices sold for non-clinical or “neuroenhancement” purposes (see [91] for review) and is especially relevant in the context of CYP with psychiatric disorders (see [92] for review).
Overall, inconsistent or unexpected findings (i.e. [38, 46, 57, 58]) underline the need to improve understand the underlying biophysiological mechanisms of tDCS, as well as how different parameters (e.g., stimulation intensity) interact with the stimulated tissue of a developing brain. One way to address this and identify optimal parameters is for future studies to broaden outcome measures to capture potential unintended effects on regions functionally related to target areas, and to explore the parameter space ideally using Bayesian optimisation (e.g. [93]), focal forms of tDCS (e.g., HD-TDCS), or open-source computational modelling software (e.g., ROAST; [94]).
Limitations
Interpretation is constrained by methodological limitations present in included studies. Heterogeneity in study design, outcome measures, stimulation protocols, and participant characteristics (e.g., age, gender, disorder profile) limited comparisons across findings and adequately powered meta-analyses of clinical, cognitive, or mood outcomes. All open-label studies and case reports or series were rated as poor quality, while RCTs had some concerns (n = 6) or a high (n = 7) risk of bias. These ratings were mainly due to a lack of detail regarding randomisation and allocation concealment. Only 17 studies performed any statistical analysis on outcome measures, and of those studies that reported statistically significant effects, four were open-label or case series/reports. Across studies, one [50] corrected for multiple testing and three [40, 48, 50] assessed integrity of blinding of parents, raters and/or experimenters, i.e., it cannot be ruled out that effects were due to placebo or test–retest effects, false-positives, and/or bias by knowledge of group assignment [95]. Only eight studies combined stimulation with cognitive training, which has been reported to boost and prolong the effects of tDCS [96, 97]. Sample sizes were between 15 and 50, which is short of that required to detect a medium effect in cognitive tasks (e.g. [98]).
It appears that the direction of traffic is towards improving the quality of studies. This is reflected by the fact that the majority of the 11 ongoing, or upcoming, trials we identified are double-, triple-, or quadruple-blind RCTs with larger sample sizes (~ 70 on average) with half combining tDCS with cognitive training across multiple sessions. However, 8 out of 11 registered trials are recruiting children with either ASD or ADHD; thus we cannot be sure that the same improvement in study quality will be seen across other psychiatric disorders or non-registered trials.
Conclusion
Although encouraging, the evidence to date is insufficient to conclude that tDCS can improve clinical symptoms, mood, or cognition in CYP with psychiatric disorders. This is largely due to the heterogeneous study designs, limited outcomes, and small sample sizes, as these limit the interpretability and comparability of findings across studies. Future studies should seek to confirm existing findings with larger samples, and randomised, sham-controlled designs that include measures of clinical, cognitive, and mood outcomes immediately after stimulation and in longer-term follow-ups. Stimulation protocols should be justified and should consider any possible unintended outcomes that might occur, particularly in younger populations.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
References
Kieling C, Baker-Henningham H, Belfer M, Conti G, Ertem I, Omigbodun O, Rohde LA, Srinath S, Ulkuer N, Rahman A (2011) Child and adolescent mental health worldwide: evidence for action. Lancet 378(9801):1515–1525. https://doi.org/10.1016/S0140-6736(11)60827-1
Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE (2005) Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry 62(6):593. https://doi.org/10.1001/archpsyc.62.6.593
Solmi M, Radua J, Olivola M, Croce E, Soardo L, Salazar de Pablo G, Il Shin J, Kirkbride JB, Jones P, Kim JH, Kim JY, Carvalho AF, Seeman MV, Correll CU, Fusar-Poli P (2021) Age at onset of mental disorders worldwide: large-scale meta-analysis of 192 epidemiological studies. Mol Psychiatry. https://doi.org/10.1038/s41380-021-01161-7
Lees B, Squeglia LM, McTeague LM, Forbes MK, Krueger RF, Sunderland M, Baillie AJ, Koch F, Teesson M, Mewton L (2021) Altered neurocognitive functional connectivity and activation patterns underlie psychopathology in preadolescence. Biol Psychiatry: Cogn Neurosci Neuroimaging 6(4):387–398. https://doi.org/10.1016/j.bpsc.2020.09.007
Patel V (2018) Acting early: The key to preventing mental health problems. J R Soc Med 111(5):153–157. https://doi.org/10.1177/0141076818764995
Ben Amor L (2012) Antipsychotics in pediatric and adolescent patients: a review of comparative safety data. J Affect Disord 138:S22–S30. https://doi.org/10.1016/j.jad.2012.02.030
Sharma AN, Arango C, Coghill D, Gringras P, Nutt DJ, Pratt P, Young AH, Hollis C (2016) BAP position statement: off-label prescribing of psychotropic medication to children and adolescents. J Psychopharmacol 30(5):416–421. https://doi.org/10.1177/0269881116636107
McGorry PD, Mei C, Chanen A, Hodges C, Alvarez-Jimenez M, Killackey E (2022) Designing and scaling up integrated youth mental health care. World Psychiatry 21(1):61–76. https://doi.org/10.1002/wps.20938
Amminger GP, Berger M, Rice SM, Davey CG, Schäfer MR, McGorry PD (2017) Novel biotherapies are needed in youth mental health. Australas Psychiatry 25(2):117–120. https://doi.org/10.1177/1039856217698237
Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, Cohen LG, Fregni F, Herrmann CS, Kappenman ES, Knotkova H, Liebetanz D, Miniussi C, Miranda PC, Paulus W, Priori A, Reato D, Stagg C, Wenderoth N, Nitsche MA (2016) A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol 127(2):1031–1048. https://doi.org/10.1016/j.clinph.2015.11.012
Nitsche MA, Paulus W (2000) Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527(3):633–639. https://doi.org/10.1111/j.1469-7793.2000.t01-1-00633.x
Stagg CJ, Antal A, Nitsche MA (2018) Physiology of transcranial direct current stimulation. J ECT 34(3):144–152. https://doi.org/10.1097/YCT.0000000000000510
Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F, Paulus W (2003) Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol 553(1):293–301. https://doi.org/10.1113/jphysiol.2003.049916
Nitsche MA, Lampe C, Antal A, Liebetanz D, Lang N, Tergau F, Paulus W (2006) Dopaminergic modulation of long-lasting direct current-induced cortical excitability changes in the human motor cortex: dopamine in human neuroplasticity. Eur J Neurosci 23(6):1651–1657. https://doi.org/10.1111/j.1460-9568.2006.04676.x
Stagg CJ, Best JG, Stephenson MC, O’Shea J, Wylezinska M, Kincses ZT, Morris PG, Matthews PM, Johansen-Berg H (2009) Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci 29(16):5202–5206. https://doi.org/10.1523/JNEUROSCI.4432-08.2009
Krause B, Márquez-Ruiz J, Kadosh RC (2013) The effect of transcranial direct current stimulation: a role for cortical excitation/inhibition balance? Front Hum Neurosci. https://doi.org/10.3389/fnhum.2013.00602
Zheng X, Alsop DC, Schlaug G (2011) Effects of transcranial direct current stimulation (tDCS) on human regional cerebral blood flow. Neuroimage 58(1):26–33. https://doi.org/10.1016/j.neuroimage.2011.06.018
Jamil A, Batsikadze G, Kuo H, Meesen RLJ, Dechent P, Paulus W, Nitsche MA (2020) Current intensity- and polarity-specific online and aftereffects of transcranial direct current stimulation: an fMRI study. Hum Brain Mapp 41(6):1644–1666. https://doi.org/10.1002/hbm.24901
Moliadze V, Lyzhko E, Schmanke T, Andreas S, Freitag CM, Siniatchkin M (2018) 1 mA cathodal tDCS shows excitatory effects in children and adolescents: insights from TMS evoked N100 potential. Brain Res Bull 140:43–51. https://doi.org/10.1016/j.brainresbull.2018.03.018
Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, Mourdoukoutas AP, Kronberg G, Truong D, Boggio P, Brunoni AR, Charvet L, Fregni F, Fritsch B, Gillick B, Hamilton RH, Hampstead BM, Jankord R, Kirton A, Woods AJ (2016) Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul 9(5):641–661. https://doi.org/10.1016/j.brs.2016.06.004
Buchanan D, Bogdanowicz T, Khanna N, Lockman-Dufour G, Robaey P, D’Angiulli A (2021) Systematic review on the safety and tolerability of transcranial direct current stimulation in children and adolescents. Brain Sci 11(2):212. https://doi.org/10.3390/brainsci11020212
Fregni F, El-Hagrassy MM, Pacheco-Barrios K, Carvalho S, Leite J, Simis M, Brunelin J, Nakamura-Palacios EM, Marangolo P, Venkatasubramanian G, San-Juan D, Caumo W, Bikson M, Brunoni AR, Cardenas-Rojas A, Giannoni-Luza S, Leao J, Teixeira Leffa D, Zeng H (2021) Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation in neurological and psychiatric disorders. Int J Neuropsychopharmacol 24(4):256–313. https://doi.org/10.1093/ijnp/pyaa051
Moffa AH, Brunoni AR, Nikolin S, Loo CK (2018) Transcranial direct current stimulation in psychiatric disorders. Psychiatr Clin N Am 41(3):447–463. https://doi.org/10.1016/j.psc.2018.05.002
Hameed MQ, Dhamne SC, Gersner R, Kaye HL, Oberman LM, Pascual-Leone A, Rotenberg A (2017) Transcranial magnetic and direct current stimulation in children. Curr Neurol Neurosci Rep 17(2):11. https://doi.org/10.1007/s11910-017-0719-0
Lee JC, Kenney-Jung DL, Blacker CJ, Doruk Camsari D, Lewis CP (2019) Transcranial direct current stimulation in child and adolescent psychiatric disorders. Child Adolesc Psychiatr Clin N Am 28(1):61–78. https://doi.org/10.1016/j.chc.2018.07.009
Muszkat D, Polanczyk GV, Dias TGC, Brunoni AR (2016) Transcranial direct current stimulation in child and adolescent psychiatry. J Child Adolesc Psychopharmacol 26(7):590–597. https://doi.org/10.1089/cap.2015.0172
Palm U, Segmiller FM, Epple AN, Freisleder F-J, Koutsouleris N, Schulte-Körne G, Padberg F (2016) Transcranial direct current stimulation in children and adolescents: a comprehensive review. J Neural Transm 123(10):1219–1234. https://doi.org/10.1007/s00702-016-1572-z
Osório AAC, Brunoni AR (2019) Transcranial direct current stimulation in children with autism spectrum disorder: a systematic scoping review. Dev Med Child Neurol 61(3):298–304. https://doi.org/10.1111/dmcn.14104
Finisguerra A, Borgatti R, Urgesi C (2019) Non-invasive brain stimulation for the rehabilitation of children and adolescents with neurodevelopmental disorders: a systematic review. Front Psychol 10:135. https://doi.org/10.3389/fpsyg.2019.00135
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. https://doi.org/10.1136/bmj.n71
Lewis YD, Gallop L, Campbell IC, Schmidt U (2021) Effects of non-invasive brain stimulation in children and young people with psychiatric disorders: a protocol for a systematic review. Syst Rev 10(1):76. https://doi.org/10.1186/s13643-021-01627-3
Li T, Higgins JPT, Deeks JJ (editors) (2021) Chapter 5: Collecting data. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds) Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021). Cochrane. www.training.cochrane.org/handbook
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H-Y, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, Higgins JPT (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. https://doi.org/10.1136/bmj.l4898
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan A-W, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Higgins JP (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. https://doi.org/10.1136/bmj.i4919
Amatachaya A, Auvichayapat N, Patjanasoontorn N, Suphakunpinyo C, Ngernyam N, Aree-uea B, Keeratitanont K, Auvichayapat P (2014) Effect of anodal transcranial direct current stimulation on autism: a randomized double-blind crossover trial. Behav Neurol. https://doi.org/10.1155/2014/173073
Auvichayapat N, Patjanasoontorn N, Phuttharak W, Suphakunpinyo C, Keeratitanont K, Tunkamnerdthai O, Aneksan B, Klomjai W, Boonphongsathian W, Sinkueakunkit A, Punjaruk W, Tiamkao S, Auvichayapat P (2020) Brain metabolite changes after anodal transcranial direct current stimulation in autism spectrum disorder. Front Mol Neurosci 13:70. https://doi.org/10.3389/fnmol.2020.00070
Auvichayapat P, Intayot K, Udomchat C, Suphakunpinyo C, Patjanasoontorn N, Keeratitanont K, Tunkamnerdthai O, Jensen MP, Humbert AT, Auvichayapat N (2022) Long-term effects of transcranial direct current stimulation in the treatment of autism spectrum disorder: a randomized controlled trial. Dev Med Child Neurol. https://doi.org/10.1111/dmcn.15457
D’Urso G, Toscano E, Sanges V, Sauvaget A, Sheffer CE, Riccio MP, Ferrucci R, Iasevoli F, Priori A, Bravaccio C, de Bartolomeis A (2021) Cerebellar transcranial direct current stimulation in children with autism spectrum disorder: a pilot study on efficacy, feasibility, safety, and unexpected outcomes in tic disorder and epilepsy. J Clin Med 11(1):143. https://doi.org/10.3390/jcm11010143
Hadoush H, Nazzal M, Almasri NA, Khalil H, Alafeef M (2020) Therapeutic effects of bilateral anodal transcranial direct current stimulation on prefrontal and motor cortical areas in children with autism spectrum disorders: a pilot study. Autism Res 13(5):828–836. https://doi.org/10.1002/aur.2290
Han YMY, Chan MMY, Shea CKS, Lai OL, Krishnamurthy K, Cheung M, Chan AS (2022) Neurophysiological and behavioral effects of multisession prefrontal tDCS and concurrent cognitive remediation training in patients with autism spectrum disorder (ASD): a double-blind, randomized controlled fNIRS study. Brain Stimul 15(2):414–425. https://doi.org/10.1016/j.brs.2022.02.004
Qiu J, Kong X, Li J, Yang J, Huang Y, Huang M, Sun B, Su J, Chen H, Wan G, Kong J (2021) Transcranial direct current stimulation (tDCS) over the left dorsal lateral prefrontal cortex in children with autism spectrum disorder (ASD). Neural Plast 2021:1–11. https://doi.org/10.1155/2021/6627507
Wilson JE, Quinn DK, Wilson JK, Garcia CM, Tesche CD (2018) Transcranial direct current stimulation to the right temporoparietal junction for social functioning in autism spectrum disorder: a case report. J ECT 34(1):e10–e13. https://doi.org/10.1097/YCT.0000000000000445
Zemestani M, Hoseinpanahi O, Salehinejad MA, Nitsche MA (2022) The impact of prefrontal transcranial direct current stimulation (tDCS) on theory of mind, emotion regulation and emotional-behavioral functions in children with autism disorder: a randomized, sham-controlled, and parallel-group study. Autism Res 15(10):1985–2003. https://doi.org/10.1002/aur.2803
Bandeira ID, Guimarães RSQ, Jagersbacher JG, Barretto TL, de Jesus-Silva JR, Santos SN, Argollo N, Lucena R (2016) Transcranial direct current stimulation in children and adolescents with attention-deficit/hyperactivity disorder (ADHD): a pilot study. J Child Neurol 31(7):918–924. https://doi.org/10.1177/0883073816630083
Berger I, Dakwar-Kawar O, Grossman ES, Nahum M, Cohen Kadosh R (2021) Scaffolding the attention-deficit/hyperactivity disorder brain using transcranial direct current and random noise stimulation: a randomized controlled trial. Clin Neurophysiol 132(3):699–707. https://doi.org/10.1016/j.clinph.2021.01.005
Breitling-Ziegler C, Zaehle T, Wellnhofer C, Dannhauer M, Tegelbeckers J, Baumann V, Flechtner H-H, Krauel K (2021) Effects of a five-day HD-tDCS application to the right IFG depend on current intensity: a study in children and adolescents with ADHD. Progress in Brain Research, vol 264. Elsevier, pp 117–150. https://doi.org/10.1016/bs.pbr.2021.01.014
Klomjai W, Siripornpanich V, Aneksan B, Vimolratana O, Permpoonputtana K, Tretriluxana J, Thichanpiang P (2022) Effects of cathodal transcranial direct current stimulation on inhibitory and attention control in children and adolescents with attention-deficit hyperactivity disorder: a pilot randomized sham-controlled crossover study. J Psychiatr Res 150:130–141. https://doi.org/10.1016/j.jpsychires.2022.02.032
Schertz M, Karni-Visel Y, Genizi J, Manishevitch H, Lam M, Akawi A, Dudai M, Fenton AA, Bikson M (2022) Transcranial direct current stimulation (tDCS) in children with ADHD: a randomized, sham-controlled pilot study. J Psychiatr Res 155:302–312. https://doi.org/10.1016/j.jpsychires.2022.08.022
Soff C, Sotnikova A, Christiansen H, Becker K, Siniatchkin M (2017) Transcranial direct current stimulation improves clinical symptoms in adolescents with attention deficit hyperactivity disorder. J Neural Transm 124(1):133–144. https://doi.org/10.1007/s00702-016-1646-y
Westwood SJ, Criaud M, Lam S-L, Lukito S, Wallace-Hanlon S, Kowalczyk OS, Kostara A, Mathew J, Agbedjro D, Wexler BE, Cohen Kadosh R, Asherson P, Rubia K (2021) Transcranial direct current stimulation (tDCS) combined with cognitive training in adolescent boys with ADHD: a double-blind, randomised, sham-controlled trial. Psychol Med. https://doi.org/10.1017/S0033291721001859
Andrade C (2013) Once- to twice-daily, 3-year domiciliary maintenance transcranial direct current stimulation for severe, disabling, clozapine-refractory continuous auditory hallucinations in schizophrenia. J ECT 29(3):239–242. https://doi.org/10.1097/YCT.0b013e3182843866
Palm U, Keeser D, Blautzik J, Pogarell O, Ertl-Wagner B, Kupka MJ, Reiser M, Padberg F (2013) Prefrontal transcranial direct current stimulation (tDCS) changes negative symptoms and functional connectivity MRI (fcMRI) in a single case of treatment-resistant schizophrenia. Schizophr Res 150(2–3):583–585. https://doi.org/10.1016/j.schres.2013.08.043
Rakesh G, Shivakumar V, Subramaniam A, Nawani H, Amaresha AC, Narayanaswamy JC, Venkatasubramanian G (2013) Monotherapy with tDCS for schizophrenia: a case report. Brain Stimul 6(4):708–709. https://doi.org/10.1016/j.brs.2013.01.007
Shenoy S, Bose A, Chhabra H, Dinakaran D, Agarwal SM, Shivakumar V, Narayanaswamy JC, Sivakumar PT, Venkatasubramanian G (2015) Transcranial direct current stimulation (tDCS) for auditory verbal hallucinations in schizophrenia during pregnancy: a case report. Brain Stimul 8(1):163–164. https://doi.org/10.1016/j.brs.2014.10.013
Hazari N, Narayanaswamy JC, Chhabra H, Bose A, Venkatasubramanian G, Reddy YCJ (2016) Response to transcranial direct current stimulation in a case of episodic obsessive compulsive disorder. J ECT 32(2):144–146. https://doi.org/10.1097/YCT.0000000000000309
Narayanaswamy JC, Jose D, Chhabra H, Agarwal SM, Shrinivasa B, Hegde A, Bose A, Kalmady SV, Venkatasubramanian G, Reddy YCJ (2015) Successful application of add-on transcranial direct current stimulation (tDCS) for treatment of SSRI resistant OCD. Brain Stimul 8(3):655–657. https://doi.org/10.1016/j.brs.2014.12.003
Behler N, Leitner B, Mezger E, Weidinger E, Musil R, Blum B, Kirsch B, Wulf L, Löhrs L, Winter C, Padberg F, Palm U (2018) Cathodal tDCS over motor cortex does not improve tourette syndrome: lessons learned from a case series. Front Behav Neurosci 12:194. https://doi.org/10.3389/fnbeh.2018.00194
Carvalho S, Gonçalves ÓF, Soares JM, Sampaio A, Macedo F, Fregni F, Leite J (2015) Sustained effects of a neural-based intervention in a refractory case of tourette syndrome. Brain Stimul 8(3):657–659. https://doi.org/10.1016/j.brs.2014.12.008
Baliga S, Sreeraj VS, Parlikar R, Rai D, Chhabra H, Kumar V, Venkatasubramanian G (2020) Role of transcranial direct current stimulation in bipolar depression: a case report. Asian J Psychiatry 47:101873. https://doi.org/10.1016/j.ajp.2019.101873
Sreeraj VS, Bose A, Shanbhag V, Narayanaswamy JC, Venkatasubramanian G, Benegal V (2016) Monotherapy With tDCS for treatment of depressive episode during pregnancy: a case report. Brain Stimul 9(3):457–458. https://doi.org/10.1016/j.brs.2016.03.007
Sousa GRM, Galdino MKC, Machado S, Vieira ECC, Rufino JF (2021) Reduction of social anxiety symptoms with transcranial direct current stimulation: a case report. Brain Stimul 14(3):728–729. https://doi.org/10.1016/j.brs.2021.04.011
Vaclavik D, Bechor M, Foster A, Gralnik LM, Bar-Haim Y, Pine DS, Bikson M, Silverman WK, Reeb-Sutherland BC, Pettit JW (2020) Case series of transcranial direct current stimulation as an augmentation strategy for attention bias modification treatment in adolescents with anxiety disorders. Clin Psychol Spec Educ 9(3):105–126. https://doi.org/10.17759/cpse.2020090308
Alizadehgoradel J, Imani S, Nejati V, Vanderhasselt M-A, Molaei B, Salehinejad MA, Ahmadi S, Taherifard M (2021) Improved executive functions and reduced craving in youths with methamphetamine addiction: evidence from combined transcranial direct current stimulation with mindfulness treatment. Clin Psychopharmacol Neurosci 19(4):653–668. https://doi.org/10.9758/cpn.2021.19.4.653
Shariatirad S, Vaziri A, Hassani-Abharian P, Sharifi Fardshad M, Molavi N, Fitzgerald PB (2016) Cumulative and booster effects of tdcs sessions on drug cravings, lapse, and cognitive impairment in methamphetamine use disorder: a case study report: cumulative effects of tDCS on drug craving. Am J Addict 25(4):264–266. https://doi.org/10.1111/ajad.12373
Costanzo F, Menghini D, Maritato A, Castiglioni MC, Mereu A, Varuzza C, Zanna V, Vicari S (2018) New treatment perspectives in adolescents with anorexia nervosa: the efficacy of non-invasive brain-directed treatment. Front Behav Neurosci 12:133. https://doi.org/10.3389/fnbeh.2018.00133
Costanzo F, Menghini D, Casula L, Amendola A, Mazzone L, Valeri G, Vicari S (2015) Transcranial direct current stimulation treatment in an adolescent with autism and drug-resistant catatonia. Brain Stimul 8(6):1233–1235. https://doi.org/10.1016/j.brs.2015.08.009
Francis SM, Beard KL, Tseng A, Chen M, Gillick BT, Jacob S, Conelea CA (2020) Transcranial direct current stimulation for compulsivity in adolescent fraternal twins with neurodevelopmental disorders. Brain Stimul 13(4):1153–1155. https://doi.org/10.1016/j.brs.2020.05.007
Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F (2011) A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol 14(8):1133–1145. https://doi.org/10.1017/S1461145710001690
Kortteenniemi A, Lehto SM, Javadi A-H (2019) Delayed, distant skin lesions after transcranial direct current stimulation. Brain Stimul 12(1):204–206. https://doi.org/10.1016/j.brs.2018.10.018
Palm U, Keeser D, Schiller C, Fintescu Z, Reisinger E, Padberg F, Nitsche M (2008) Skin lesions after treatment with transcranial direct current stimulation (tDCS). Brain Stimul 1(4):386–387. https://doi.org/10.1016/j.brs.2008.04.003
Luckhardt C, Schütz M, Mühlherr A, Mössinger H, Boxhoorn S, Dempfle A, Salvador R, Ruffini G, Pereira HC, Castelo-Branco M, Latinus M, Bonnet-Brilhault F, Siemann J, Siniatchkin M, Ecker C, Freitag CM (2021) Phase-IIa randomized, double-blind, sham-controlled, parallel group trial on anodal transcranial direct current stimulation (tDCS) over the left and right tempo-parietal junction in autism spectrum disorder—StimAT: study protocol for a clinical trial. Trials 22(1):248. https://doi.org/10.1186/s13063-021-05172-1
Prillinger K, Radev ST, Amador de Lara G, Klöbl M, Lanzenberger R, Plener PL, Poustka L, Konicar L (2021) Repeated sessions of transcranial direct current stimulation on adolescents with autism spectrum disorder: study protocol for a randomized, double-blind, and sham-controlled clinical trial. Front Psychiatry 12:680525. https://doi.org/10.3389/fpsyt.2021.680525
Goerigk SA, Padberg F, Bühner M, Sarubin N, Kaster TS, Daskalakis ZJ, Blumberger DM, Borrione L, Razza LB, Brunoni AR (2021) Distinct trajectories of response to prefrontal tDCS in major depression: results from a 3-arm randomized controlled trial. Neuropsychopharmacology 46(4):774–782. https://doi.org/10.1038/s41386-020-00935-x
Davidson RJ, Lewis DA, Alloy LB, Amaral DG, Bush G, Cohen JD, Drevets WC, Farah MJ, Kagan J, McClelland JL, Nolen-Hoeksema S, Peterson BS (2002) Neural and behavioral substrates of mood and mood regulation. Biol Psychiat 52(6):478–502. https://doi.org/10.1016/S0006-3223(02)01458-0
Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF (2007) Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiat 62(5):429–437. https://doi.org/10.1016/j.biopsych.2006.09.020
Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, Rafi-Tari S (2004) Limbic–frontal circuitry in major depression: a path modeling metanalysis. Neuroimage 22(1):409–418. https://doi.org/10.1016/j.neuroimage.2004.01.015
Telzer EH, Mogg K, Bradley BP, Mai X, Ernst M, Pine DS, Monk CS (2008) Relationship between trait anxiety, prefrontal cortex, and attention bias to angry faces in children and adolescents. Biol Psychol 79(2):216–222. https://doi.org/10.1016/j.biopsycho.2008.05.004
Katz B, Au J, Buschkuehl M, Abagis T, Zabel C, Jaeggi SM, Jonides J (2017) Individual differences and long-term consequences of tDCS-augmented cognitive training. J Cogn Neurosci 29(9):1498–1508. https://doi.org/10.1162/jocn_a_01115
Wiegand A, Sommer A, Nieratschker V, Plewnia C (2019) Improvement of cognitive control and stabilization of affect by prefrontal transcranial direct current stimulation (tDCS). Sci Rep 9(1):6797. https://doi.org/10.1038/s41598-019-43234-2
McIntyre RS, Cha DS, Soczynska JK, Woldeyohannes HO, Gallaugher LA, Kudlow P, Alsuwaidan M, Baskaran A (2013) Cognitive deficits and functional outcomes in major depressive disorder: determinants, substrates, and treatment interventions: review: cognitive deficits and functional outcomes in MDD. Depress Anxiety 30(6):515–527. https://doi.org/10.1002/da.22063
Shamsi S, Lau A, Lencz T, Burdick KE, DeRosse P, Brenner R, Lindenmayer J-P, Malhotra AK (2011) Cognitive and symptomatic predictors of functional disability in schizophrenia. Schizophr Res 126(1–3):257–264. https://doi.org/10.1016/j.schres.2010.08.007
Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos-Quiroga JA, Rohde LA, Sonuga-Barke EJS, Tannock R, Franke B (2015) Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers 1(1):15020. https://doi.org/10.1038/nrdp.2015.20
Craig F, Margari F, Legrottaglie A, Palumbi R, De Giambattista C, Margari L (2016) A review of executive function deficits in autism spectrum disorder and attention-deficit/hyperactivity disorder. Neuropsychiatr Dis Treat. https://doi.org/10.2147/NDT.S104620
van Duin D, van Wamel A, de Winter L, Kroon H, Veling W, van Weeghel J (2021) Implementing evidence-based interventions to improve vocational recovery in early psychosis: a quality-improvement report. Psychiatr Serv 72(10):1168–1177. https://doi.org/10.1176/appi.ps.201900342
Kirkpatrick B (2014) Developing concepts in negative symptoms: primary vs secondary and apathy vs expression. J Clin Psychiatry 75(suppl 1):3–7. https://doi.org/10.4088/JCP.13049su1c.01
Bessonova L, Velligan DI, Weiden PJ, O’Sullivan AK, Yarlas A, Bayliss M, Baranwal N, Rychlec K, Carpenter-Conlin J, Doane MJ, Sajatovic M (2020) Antipsychotic treatment experiences of people with bipolar I disorder: patient perspectives from an online survey. BMC Psychiatry 20(1):354. https://doi.org/10.1186/s12888-020-02767-x
van Boekholdt L, Kerstens S, Khatoun A, Asamoah B, Mc Laughlin M (2021) tDCS peripheral nerve stimulation: a neglected mode of action? Mol Psychiatry 26(2):456–461. https://doi.org/10.1038/s41380-020-00962-6
Minhas P, Bikson M, Woods AJ, Rosen AR, Kessler SK (2012) Transcranial direct current stimulation in pediatric brain: a computational modeling study. In:2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 859–862. https://doi.org/10.1109/EMBC.2012.6346067
Kessler SK, Minhas P, Woods AJ, Rosen A, Gorman C, Bikson M (2013) Dosage considerations for transcranial direct current stimulation in children: a computational modeling study. PLoS ONE 8(9):e76112. https://doi.org/10.1371/journal.pone.0076112
Morya E, Monte-Silva K, Bikson M, Esmaeilpour Z, Biazoli CE, Fonseca A, Bocci T, Farzan F, Chatterjee R, Hausdorff JM, da Silva-Machado DG, Brunoni AR, Mezger E, Moscaleski LA, Pegado R, Sato JR, Caetano MS, Sá KN, Tanaka C, Okano AH (2019) Beyond the target area: an integrative view of tDCS-induced motor cortex modulation in patients and athletes. J NeuroEng Rehabil 16(1):141. https://doi.org/10.1186/s12984-019-0581-1
Day P, Twiddy J, Dubljević V (2023) Present and emerging ethical issues with tDCS use: a summary and review. Neuroethics 16(1):1. https://doi.org/10.1007/s12152-022-09508-9
Sierawska A, Prehn-Kristensen A, Moliadze V, Krauel K, Nowak R, Freitag CM, Siniatchkin M, Buyx A (2019) Unmet needs in children with attention deficit hyperactivity disorder—can transcranial direct current stimulation fill the gap? Promises and ethical challenges. Front Psych 10:334. https://doi.org/10.3389/fpsyt.2019.00334
Lorenz R, Johal M, Dick F, Hampshire A, Leech R, Geranmayeh F (2021) A Bayesian optimization approach for rapidly mapping residual network function in stroke. Brain 144(7):2120–2134. https://doi.org/10.1093/brain/awab109
Huang Y, Datta A, Bikson M, Parra LC (2018) ROAST: an open-source, fully-automated, realistic volumetric-approach-based simulator for TES. In: 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 3072–3075. https://doi.org/10.1109/EMBC.2018.8513086
Forbes D (2013) Blinding: an essential component in decreasing risk of bias in experimental designs. Evid Based Nurs 16(3):70–71. https://doi.org/10.1136/eb-2013-101382
Antal A, Terney D, Poreisz C, Paulus W (2007) Towards unravelling task-related modulations of neuroplastic changes induced in the human motor cortex: effect of tDCS is modified by mental activity and exercise. Eur J Neurosci 26(9):2687–2691. https://doi.org/10.1111/j.1460-9568.2007.05896.x
Burton C, Wright B, Hampstead B, Tso I, Taylor S (2020) Combining tDCS and cognitive training for people with severe mental illness: preliminary findings. Biol Psychiat 87(9):S263. https://doi.org/10.1016/j.biopsych.2020.02.680
Minarik T, Berger B, Althaus L, Bader V, Biebl B, Brotzeller F, Fusban T, Hegemann J, Jesteadt L, Kalweit L, Leitner M, Linke F, Nabielska N, Reiter T, Schmitt D, Spraetz A, Sauseng P (2016) The importance of sample size for reproducibility of tDCS effects. Front Hum Neurosci. https://doi.org/10.3389/fnhum.2016.00453
Acknowledgements
LG was supported by a PhD studentship from the National Institute of Health Research (NIHR) Biomedical Research Centre (BRC) for Mental Health, South London and Maudsley NHS Foundation Trust (SLaM) and Institute of Psychiatry, Psychology and Neuroscience (IoPPN) at King's College London (KCL). YDL was supported by the Daniel Turnberg Royal College of Physicians travel fellowship. This work is supported by the MRC/AHRC/ESRC Adolescence, Mental Health and the Developing Mind initiative as part of the EDIFY programme (grant number MR/W002418/1). US and ICC receive salary support from the NIHR BRC for Mental Health, SLaM, and Institute of Psychiatry, Psychology and Neuroscience, KCL. The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the NIHR, or the UK Department of Health.
Author information
Authors and Affiliations
Contributions
LG, YDL, ICC, and US contributed to the design of the review. LG and YL did the search, screening, data extraction and quality assessment. LG and SJW did the update search, screening, data extraction and quality assessment. LG reviewed the included studies and wrote the draft report. LG, SJW, ICC, and US contributed to revising consecutive drafts. All authors reviewed the review findings and approved the final version before submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflict of interests.
Ethical approval
No ethical approval was required.
Permission
We have not used figures from other sources or that have been published elsewhere.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gallop, L., Westwood, S.J., Lewis, Y. et al. Effects of transcranial direct current stimulation in children and young people with psychiatric disorders: a systematic review. Eur Child Adolesc Psychiatry (2023). https://doi.org/10.1007/s00787-023-02157-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00787-023-02157-0