Abstract
Objectives
Management of the neck in patients with oral squamous cell carcinoma (OSCC) is pivotal to oncologic control and survival. However, there is controversy regarding necessity of neck dissection (ND) in patients with clinically node-negative neck. We aimed to assess risk factors for occult metastasis and to explore whether the presence of occult lymph node metastases (LNMs) has an impact on recurrence and survival.
Material and methods
A retrospective cohort study was performed including patients with primary OSCC who underwent radical tumor resection and ND in a high-volume center adhering to the prevailing German guideline. The ND was performed according to a standardized approach.
Results
Four hundred twenty-one patients with primary surgically treated OSCC were included. The incidence of occult metastasis was 14.49%. A pathological T stage > 1 (multivariate analysis, odds ratio (OR) 3.958, p = 0.042) and the presence of extranodal extension in LNMs (multivariate analysis, OR 0.287, p = 0.020) were identified as independent risk factors for occult metastasis. When comparing patients with and without occult metastasis, there were no significant differences in terms of progression-free survival (log-rank, p = 0.297) and overall survival (log-rank, p = 0.320). There were no cases of ipsilateral neck recurrence. One patient developed contralateral neck metastasis; however, he initially presented with a unilateral pT1 pN0 tumor.
Conclusions
Overall, our findings suggest that conducting a standardized approach in ND should be applied in terms of management of the neck in order to maintain survival rates and to prevent neck recurrence in OSCC patients.
Clinical relevance.
None of the risk factors for occult metastasis can be reliably assessed preoperatively. Although elective ND does not guarantee the complete prevention of neck recurrence, it increases the likelihood of either timely removal of micrometastases or strengthens the justification for adjuvant therapy. Consequently, this approach leads to improvements in clinical outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral squamous cell carcinoma (OSCC) accounts for approximately 90% of all malignant tumors within the oral cavity, with a global incidence surpassing 350,000 cases [1, 2].
OSCC is characterized by a high propensity for cervical lymph node metastases (LNMs), affecting approximately 42.6% of the patients [3]. As dissemination to the regional lymph nodes represents the most critical prognostic factor in OSCC patients [4], effective management of the neck is indispensable for oncological control and survival [5]. Despite this, there has been ongoing controversy regarding the optimal approach for patients without clinically detectable LNMs—a debate that has persisted since the 1980s [6].
The prevalence of occult nodal metastasis in clinically node-negative (cN0) necks varies from 7.3 to 36.8% [7, 8], and their progression to clinically evident LNMs is known to be associated with poor oncological outcomes [9]. The proportion of occult metastases naturally depends on the sensitivity of the preoperative clinical examination and the employed diagnostic modalities, e.g., computed tomography or magnetic resonance imaging, as well as the definition of pathological LNMs in imaging [10].
In 1994, Weiss et al. [11] were the first to propose a threshold for recommending ND based on the percentage of occult metastases. They indicated that patients with a risk of occult metastasis greater than 20% have improved regional control, disease-specific survival, and overall survival when undergoing ND [11]. As a general rule of thumb, many clinicians today recommend elective ND when the risk of occult LNM exceeds 15–20% [12, 13].
The primary rationale for advocating routine elective neck dissection (ND) as part of the primary treatment of OSCC patients is the early detection of occult metastasis, enabling adjustments to the adjuvant treatment plan and improving prognosis [14, 15]. However, ND is associated with potential morbidity, such as shoulder pain and dysfunction due to accessory nerve paralysis [13], prompting exploration into less invasive alternatives such as sentinel lymph node biopsy (SNB) or a wait-and-see policy for treatment deintensification.
The objectives of this present study were twofold: first, to identify the risk factors associated with occult metastasis, and second, to investigate whether the presence of occult LNMs has an impact on recurrence along with progression-free survival (PFS) and overall survival (OS) when ND is performed according to a standardized approach.
Methods
Study design and participants
The study cohort encompassed patients with primary OSCC, who received treatment including radical tumor resection and ND. The treatment regime adhered to the prevailing German guidelines and was conducted in a high-volume center between January 1, 2013, and May 31, 2023. All treatments were performed according to oncology board meetings’ recommendations.
The ND procedure followed a standardized approach as shown in Fig. 1. We consistently performed split-up NDs, as this approach involves dissecting lymph node specimens into packages, allowing for the categorization of LNMs into cervical levels following histopathological analysis [16, 17]. This information empowers clinicians to make decisions about whether to extend the ND to levels IV and V, and to tailor adjuvant radiotherapy [16].
The necessity of adjuvant radiotherapy or radiochemotherapy was determined based on the individual risk factors of each patient, in accordance with the recommendations set forth in the German guideline.
The follow-up schedule was as follows: in the first year, clinical examinations were performed every 6 weeks; in the second year, it was every 3 months; during the third and fourth years, follow-ups were scheduled every 6 months, and in the fifth year, assessments took place after 12 months. Additional computed tomography scans were conducted every 6 months in the first 2 years and every 12 months for the subsequent 3 years.
Exclusion criteria encompassed recurrent OSCC and squamous cell carcinoma of the lip. Additionally, patients who either declined to undergo ND or had a reduced extent of ND due to severe comorbidities were excluded. Moreover, to prevent potential bias stemming from surgery-related short-term mortality, patients who passed away within 30 days following surgery (perioperative death) were excluded from survival analyses. Patients with a follow-up period of less than 30 days were also excluded.
The study’s design and methodologies received approval from the Ethics Committee of the Friedrich-Alexander-University Erlangen-Nuremberg (Ethic votes: 23–185-Br, 23–186-Br). In accordance with national and institutional regulations, written informed consent was not necessary.
The manuscript was prepared according to the STROBE statement.
Contrast-enhanced computed tomography
Prior to surgery, all patients included in this study underwent preoperative thin-section axial multidetector computed tomography scans for staging. These scans were conducted using an intravenous iodine-based contrast agent to enhance soft tissue differentiation. The assessment of imaging data involved a minimum of two independent physicians from the Department of Radiology. At least one consultant assessed the local extent of the tumor and evaluated the lymph node status.
Clinicopathological characteristics
Clinicopathological characteristics were obtained from the clinical hospital files. The following parameters were systematically recorded and evaluated: age, sex, tumor localization, TNM classification, depth of invasion (DOI), histological grading, resection margins, presence of perineural, vascular, and lymphovascular invasion, and extranodal extension (ENE). In addition, time point of surgery and time point of last follow-up as well as time point of death were recorded.
The TNM classification was revised during the study period. To ensure the consistency of our results, we restaged patients who were initially classified using the 7th TNM classification. Thereby, all patients were classified according to the 8th TNM classification.
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences 28.0 (SPSS, Chicago, IL, USA).
Correlation analysis was performed using chi-square test.
For evaluating risk factors of occult metastasis, we utilized logistic regression analysis, followed by a multivariate analysis that incorporated factors showing significance in the univariate analysis.
In addition, PFS and OS in patients with and without occult metastasis were estimated using the Kaplan–Meier method. We utilized the log-rank test to compare survival outcomes between the two groups.
PFS was defined as the time elapsed from the day of surgery to locoregional or lymph node/distant metastatic recurrence and was censored on the last day when the patient was alive without any evidence of recurrence. OS was defined as the time from the day of resection to death from any cause and was censored at the last day when the patient was alive.
Figures were also created using SPSS.
Generally, a p value < 0.05 was considered statistically significant.
Results
Our final study cohort compromised 421 patients with primary OSCC treated with radical tumor resection and ND.
Patients’ clinicopathological characteristics are detailed in Table 1.
The median age of the patient cohort was 64 years, with a range between 31 and 93 years. The included patients were predominantly male (260/421, 61.76%) and the majority of the tumors were localized either at the floor of the mouth (150/421, 35.63%) or at the tongue (105/421, 24.94%).
Further analysis revealed that 175 (41.57%) of the tumors exhibited a DOI of 5 mm or less, 112 (26.60%) fell within the 6–10 mm range, and 101 (23.99%) had a DOI exceeding 10 mm.
Overall, the incidence of LNMs was 33.97%, encompassing 143 out of the 421 patients. Of these patients, 58 (constituting 40.56%) presented LNMs accompanied with ENE. Occult metastases were identified in 61 out of the 421 patients, equating to a rate of 14.49% (see Table 4). 9.26% (39/421) of OSCC patients were falsely diagnosed with nodal disease preoperatively (false positive rate).
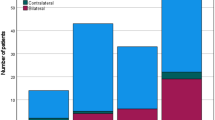
Approximately half of the patients (29/61) with occult metastasis exhibited a single LNM, classifying them as N1 (representing 47.54%). An additional 13.11% of patients had a single LNM with ENE (8 out of 61 patients). Moreover, 21.31% of patients had multiple LNMs ipsilateral without ENE, leading to their classification as N2b (13 out of 61 patients). Furthermore, 4.92% (3 out of 61) and 13.11% (8 out of 61) of patients were categorized as N2c because of bilateral or contralateral metastasis, and N3b due to the presence of ENE along with more than one LNM. The distribution of N staging among patients with occult metastasis is depicted in Fig. 2.
In this study, 60.81% (256 out of 421) of the patients received adjuvant treatment, such as brachytherapy, radiotherapy, or radiochemotherapy. However, 29 patients (6.89%) chose to either decline adjuvant therapy or did not complete it, although it was recommended.
Correlation between clinicopathological characteristics and occult metastasis
Several factors were significantly associated with the presence of occult metastasis. These factors included pathological T stage (chi-square, p < 0.001), pathological N stage (chi-square, p < 0.001), presence of ENE (chi-square, p < 0.001), lymphovascular (chi-square, p = 0.010) and perineural invasion (chi-square, p = 0.002), grading (chi-square, p = 0.015), and DOI (chi-square, p < 0.001). The results of the correlation analysis are depicted in Table 2.
Risk factors for occult metastasis
Furthermore, we conducted univariate and multivariate analyses to identify risk factors for occult metastasis.
In the univariate logistic regression analysis, several factors emerged as prognostic indicators for the presence of occult metastasis. Specifically, pathological T stage greater than 1 (odds ratio (OR): 2.961, 95% CI 1.491–5.880, p = 0.002), pathological N stage greater than 1 (OR 4.738, 95% CI 4.738, p < 0.001), and the presence of perineural invasion (OR 2.506, 95% CI 1.383–4.544, p = 0.002) were identified as significant factors. Moreover, patients with lymph node metastases exhibiting ENE were found to be less likely to have occult LNMs (OR 0.276, 95% CI 0.132–0.577, p < 0.001).
Subsequently, the multivariate analysis confirmed that pathological T stage greater than 1 (OR 3.958, 95% CI 1.048–14.944, p = 0.042) and the presence of ENE (OR 0.287, 95% CI 0.118–0.698, p = 0.020) are independent risk factors for occult metastasis.
Detailed results of both the univariate and multivariate analyses can be found in Table 3.
Nodal metastases and their occult percentage depending on tumor subsite and pathological T stage
In the next step, we analyzed the prevalence of occult metastasis while considering tumor localization and pathological T stage. For all T1 tumors, regardless of their localization, the frequency of occult metastasis was approximately 7%.
Conversely, among T2 tumors, the frequency of occult metastasis exceeded 20% for all tumor subsites, with the exception of the hard palate (1 out of 8, 12.50%).
Data regarding the incidence of occult metastasis, both in total and based on tumor subsite and T stage, can be found in Table 4.
Association between depth of invasion and the occurrence of occult metastasis
The percentage of occult metastasis in patients with tumors exhibiting a DOI of 3, 4, and 5 mm was 22.22% (6/27), 8.11% (3/37), and 19.35% (6/31), respectively. Overall, the percentage of occult metastasis was 8.57% (15/175) in tumors ≤ 5 mm. Furthermore, the frequency of occult metastasis was 20.54% (23/112) and 20.79% (21/101) in tumors with DOIs between 6–10 and > 11 mm.
Data regarding the incidence of occult metastasis depending on DOI can be found in Table 5.
Survival analysis
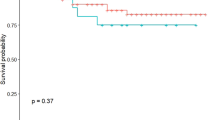
We conducted a survival analysis to compare the survival outcomes between patients with occult metastasis and those without. In our patient cohort, there were no significant differences in terms of PFS (log-rank, p = 0.297) and OS (log-rank, p = 0.320) between these two groups.
The corresponding Kaplan–Meier curves are displayed in Fig. 3.
Analysis of recurrence
Among our study cohort, 37 patients experienced local recurrence (8.79%) and 6 patients presented with simultaneous occurrences of a local recurrence along with cervical metastases (1.42%). Notably, there were no instances of isolated ipsilateral cervical metastases during the follow-up. One patient, who initially exhibited a unilateral pT1 pN0 tumor, developed contralateral neck metastasis approximately 1 year after the primary surgery (0.24%). Additionally, 15 patients (3.56%) developed distant metastases without any concomitant local or regional recurrence.
Discussion
OSCC is characterized by a high propensity for cervical LNMs, affecting approximately 42.6% of patients [3]. As dissemination to the regional lymph nodes represents the most critical prognostic factor [4], effective management of the neck is indispensable for oncological control and survival [5].
Nonetheless, there remains an ongoing debate regarding the optimal approach for patients without clinical evidence of metastasis. This debate centers on whether to adopt a wait-and-see policy, utilize less invasive techniques such as SNB, or choose elective ND, particularly in cases involving early-stage tumors.
The objectives of the present study were to evaluate the risk factors associated with occult metastasis and to investigate whether the presence of occult LNMs has an impact on recurrence and survival when ND is conducted adhering to a standardized approach.
Four hundred twenty-one patients with primary surgically treated OSCC were included in the study. Our patient cohort exhibited an incidence of cervical metastasis of 33.97% with 14.49% of patients exhibiting occult metastasis.
Previously, factors such as perineural invasion, lymphovascular invasion, DOI [18], tumor budding [19], and tumor thickness [20] have all been described to be associated with heightened rates of occult metastasis in OSCC patients [21, 22].
When evaluating predictors of occult metastasis among our study cohort, univariate logistic regression revealed pathological T stage > 1 (logistic regression, p = 0.002), pathological N stage > 1 (logistic regression, p < 0.001), and presence of perineural invasion (logistic regression, p = 0.002) as prognostic factors regarding the presence of occult metastasis. In addition, patients with ENE were less likely to have occult metastasis (logistic regression, p < 0.001). Multivariate analysis confirmed pathological T stage > 1 (multivariate analysis, p = 0.042) and presence of ENE (multivariate analysis, p = 0.020) as independent factors.
However, the disadvantage of all these factors, as mentioned above and as evident in our analysis, is that none of them can be reliably assessed before the radical tumor resection and removal of LNM, thereby significantly diminishing their clinical utility in evaluating the preoperative lymph node status.
Analyzing the prevalence of occult metastasis while considering tumor localization and T stage, it was found that the frequency remained consistently at approximately 7% for T1 tumors, regardless of the tumor’s localization within the oral cavity. Conversely, for T2 tumors, the frequency of occult metastasis exceeded 20%, except for those located at the hard palate. Nevertheless, the relatively small number of patients with carcinomas of the hard palate who were included in this study could account for this particular result. In summary, our findings suggest that all tumor localizations within the oral cavity carry a similar risk of presenting occult metastasis.
In similarity with our results, Yang et al. [23] reported an incidence of occult metastasis at 10.9% in patients with T1 tumors localized at the tongue. However, they observed a substantially higher incidence of 28.6% for those with T2 tumors [23]. Furthermore, Hutchison et al. [24] reported even higher rates, with 20.8% for T1 tumors and 36.0% for T2 tumors.
Regarding the T stage, it is noteworthy that the TNM classification underwent a substantial revision in 2017, incorporating changes that include the consideration of DOI and ENE as criteria for determining the T and N stages in patients with OSCC. As a result, conclusions drawn from the 7th TNM classification (which is relevant to both of the aforementioned studies [23, 24]) or studies involving a heterogeneous patient population may no longer provide dependable insights when assessing the occurrence of occult metastasis across various T stages.
Within our patient cohort, the risk of occult metastasis was at 22.22%, 8.11%, and 19.35% for tumors with a DOI of 3, 4, and 5 mm, respectively. In a broader context, the overall rate of occult metastasis was 8.57% for tumors with a DOI of 5 mm or less. Moreover, the incidence of occult metastasis was notably higher at 20.54% and 20.79% for tumors with DOIs falling in the range of 6–10 mm and exceeding 11 mm, respectively.
de Matos et al. [25] reported that among patients with pathological DOIs of 10 mm or less, the proportion of occult LNMs was 15.3%, whereas for those with pathological DOIs exceeding 10 mm, the proportion was substantially higher at 54.2%. They also determined a cutoff value of 10 mm for DOI as a prognostic factor [25].
Kane et al. [26] emphasized that DOI is the most significant histopathological predictor of occult metastasis in OSCC patients and that tumors with a DOI of 5 mm or more are at a heightened risk of nodal metastasis. Considering these findings, some surgeons prefer to use SNB or opt for a wait-and-see approach instead of conducting elective ND when the DOI falls within the 2 to 4 mm range in order to minimize surgical morbidity [27]. Nevertheless, there remains an ongoing debate about the precise DOI threshold that should trigger the decision to opt for elective ND.
The National Comprehensive Cancer Network guideline recommends elective ND when the DOI exceeds 3 mm [28]. On the contrary, Schilling et al. [29] even propose the potential utility of SNB for DOIs up to 10 mm.
It is essential to emphasize that the body of data concerning SNB remains somewhat limited, particularly within the context of prospective studies. A significant limitation in many previous investigations regarding SNB has been the inconsistent histological examination applied to non-sentinel lymph nodes. Furthermore, the reported success rates exhibit significant variation. For instance, Guerlain et al. reported a success rate of 93%, whereas in other studies, the reported success rates frequently fall well below 80% [30,31,32]. In addition, SNB is not suitable for all tumors, particularly those located at the floor of the mouth [33]. This limitation arises from the challenges associated with the “shine-through phenomenon” [34].
ENE holds substantial prognostic significance and serves as a pivotal factor in the risk assessment of OSCC patients, including considerations for adjuvant therapy [35]. In our analysis, patients with LNMs with ENE were less likely to present with occult metastasis (multivariate analysis, p = 0.020). However, the preoperative assessment of ENE is limited in terms of sensitivity and thereby making it impractical to make reliable determinations about its presence before surgery [36, 37].
The association of perineural invasion (p = 0.002) with occult LNMs in univariate analysis suggests that elective ND should be considered when this histopathological feature is present, even in the absence of other high-risk histopathologic features. Nonetheless, the perineural invasion did not yield statistical significance in the multivariate analysis (p = 0.408).
Occult metastasis was previously described as a burden factor in OSCC patients [38, 39]. However, within our patient cohort, no significant differences were found between patients with and without occult metastasis in terms of PFS (log-rank, p = 0.297) and OS (log-rank, p = 0.320).
Haidari et al. [38] conducted a study examining the impact of occult metastasis on survival and found that the presence of occult metastasis had a negative influence on PFS in OSCC patients (hazard ratio = 2.33). However, in contrast to our results, they reported a much lower prevalence of occult metastasis of 7.08% with a significantly higher false positive rate of 23.45% [38]. Broglie et al. [39] found similar results with occult metastasis resulting in decreased overall survival and disease-free survival. However, they employed SNB, potentially resulting in missed cases of LNMs [39].
Several studies have examined the disparity in survival outcomes and rates of recurrence between elective and therapeutic ND following a wait-and-see strategy. The results by Fasunla et al. [12] and D’Cruz et al. [13] revealed that elective ND was associated with significantly higher rates of OS and disease-free survival. Furthermore, D’Cruz et al. [13] reported that a greater proportion of patients received adjuvant radiotherapy based on nodal indications following elective neck dissection. In contrast, findings by Liu et al. [40, 41] suggested that adopting a wait-and-see policy does not appear to compromise survival.
This contradicts the findings of several trials indicating that a substantial proportion of patients with early OSCC who undergo a wait-and-see policy will develop neck recurrences, many of which will present with advanced stages and unfavorable prognostic factors like ENE [42]. For example, D’Cruz documented a neck recurrence rate of 45% [26], while Nieuwenhuis et al. [44] and Flach et al. [43] reported lymph node recurrence rates of 21% and 28%, respectively. Ho et al. [45] documented that the survival rates for these patients with recurrences were merely 30%. The elevated recurrence rates could be attributed to the presence of micrometastases (metastases smaller than 2 mm) preoperatively, which are challenging to assess due to the limitations imposed by the slice thickness in image-based techniques.
In this context, it is worth noting that in our patient cohort, there were no instances of ipsilateral LNMs observed during the follow-up period. One patient experienced contralateral neck metastasis approximately 1 year after the initial surgery (0.2%); notably, this patient initially presented with a unilateral pT1 pN0 tumor.
In contemporary medical practice, the pursuit of less invasive techniques to preserve the postoperative quality of life for patients is undoubtedly a priority. To avoid potential morbidity, some surgeons decline ND in the early stages of OSCC.
Certain anatomical structures, including the ramus marginalis n. facialis, accessory nerve, hypoglossal nerve, vagus nerve, and lingualis nerve, are susceptible to injury and potential morbidity during ND [46]. Nevertheless, elective, supraomohyoid ND typically allows for the preservation of these structures in nearly all cases, with accidental injuries being a rare occurrence [47]. On the contrary, these structures can also be at risk during SNB. Schiefke et al. [48] even described similar limitations regarding the quality of life between patients treated with SND and elective ND.
In situations involving the radical resection of advanced LNMs with ENE due to recurrence of the neck, preserving these structures is often not feasible, leading to inevitable compromises in the patient’s quality of life [47]. As described before, the likelihood of advanced LNMs is higher when a wait-and-see approach is chosen over elective ND in cN0 neck patients [42]. As a result, elective ND usually has a lower level of invasiveness compared to therapeutic ND and is thereby associated with less impairment of quality of life.
Limitations
The main limitations of this study are the sample size and the retrospective methodology. Although previous studies have examined rates of occult LNMs in OSCC, most had smaller sample sizes and contained heterogeneous data.
Conclusions
In conclusion, our findings suggest that conducting a standardized approach in ND should be applied in terms of the management of the neck in order to maintain survival rates and to prevent neck recurrence in OSCC patients. None of the risk factors for occult metastasis can be reliably assessed preoperatively. While elective ND may not guarantee the prevention of late metastases, it does enhance the likelihood of either timely removal of micrometastases or strengthens the justification for adjuvant therapy, ultimately leading to improved clinical outcomes.
In contemporary medical practice, the pursuit of less invasive techniques to preserve the postoperative quality of life for patients is undoubtedly a priority. However, it is currently inadvisable to select approaches like sentinel SNB or a wait-and-see policy solely with the intention of minimizing immediate morbidity. Such choices may heighten the risk of developing advanced lymph node disease at a later stage, which, in turn, could result in a less favorable prognosis and increased morbidity when therapeutic ND becomes necessary.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- CI:
-
Confidence interval
- cN0:
-
Clinically node negative
- DOI:
-
Depth of invasion
- ENE:
-
Extranodal extension
- OSCC:
-
Oral squamous cell carcinoma
- LNM:
-
Lymph node metastasis
- N stage:
-
Nodal stage
- OR:
-
Odds ratio
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- SNB:
-
Sentinel lymph node biopsy
- TNM:
-
Tumor, nodus, metastasis
- T stage:
-
Tumor stage
References
Massano J, Regateiro FS, Januario G, Ferreira A (2006) Oral squamous cell carcinoma: review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 102:67–76. https://doi.org/10.1016/j.tripleo.2005.07.038
Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M, Znaor A, Bray F (2019) Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 144:1941–1953. https://doi.org/10.1002/ijc.31937
Moratin J, Horn D, Metzger K, Ristow O, Flechtenmacher C, Engel M, Hoffmann J, Freier K, Freudlsperger C (2020) Squamous cell carcinoma of the mandible - patterns of metastasis and disease recurrence in dependence of localization and therapy. J Craniomaxillofac Surg 48:1158–1163. https://doi.org/10.1016/j.jcms.2020.10.006
Ganly I, Patel S, Shah J (2012) Early stage squamous cell cancer of the oral tongue–clinicopathologic features affecting outcome. Cancer 118:101–111. https://doi.org/10.1002/cncr.26229
Crile G (1906) III. On the technique of operations upon the head and neck. Ann Surg 44:842–850. https://doi.org/10.1097/00000658-190612000-00003
Vandenbrouck C, Sancho-Garnier H, Chassagne D, Saravane D, Cachin Y, Micheau C (1980) Elective versus therapeutic radical neck dissection in epidermoid carcinoma of the oral cavity: results of a randomized clinical trial. Cancer 46:386–390. https://doi.org/10.1002/1097-0142(19800715)46:2%3c386::aid-cncr2820460229%3e3.0.co;2-9
Huang SF, Chang JT, Liao CT, Kang CJ, Lin CY, Fan KH, Wang HM, Chen IH (2015) The role of elective neck dissection in early stage buccal cancer. Laryngoscope 125:128–133. https://doi.org/10.1002/lary.24840
Ebrahimi A, Ashford BG, Clark JR (2012) Improved survival with elective neck dissection in thick early-stage oral squamous cell carcinoma. Head Neck 34:709–716. https://doi.org/10.1002/hed.21809
Cai H, Zhu Y, Wang C, Zhang Y, Hou J (2020) Neck nodal recurrence and survival of clinical T1–2 N0 oral squamous cell carcinoma in comparison of elective neck dissection versus observation: a meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol 129:296–310. https://doi.org/10.1016/j.oooo.2019.10.012
Struckmeier AK, Yekta E, Agaimy A, Kopp M, Buchbender M, Moest T, Lutz R, Kesting M (2023) Diagnostic accuracy of contrast-enhanced computed tomography in assessing cervical lymph node status in patients with oral squamous cell carcinoma. J Cancer Res Clin Oncol. https://doi.org/10.1007/s00432-023-05470-y
Weiss MH, Harrison LB, Isaacs RS (1994) Use of decision analysis in planning a management strategy for the stage N0 neck. Arch Otolaryngol Head Neck Surg 120:699–702. https://doi.org/10.1001/archotol.1994.01880310005001
Fasunla AJ, Greene BH, Timmesfeld N, Wiegand S, Werner JA, Sesterhenn AM (2011) A meta-analysis of the randomized controlled trials on elective neck dissection versus therapeutic neck dissection in oral cavity cancers with clinically node-negative neck. Oral Oncol 47:320–324. https://doi.org/10.1016/j.oraloncology.2011.03.009
D’Cruz AK, Vaish R, Kapre N, Dandekar M, Gupta S, Hawaldar R, Agarwal JP, Pantvaidya G, Chaukar D, Deshmukh A, Kane S, Arya S, Ghosh-Laskar S, Chaturvedi P, Pai P, Nair S, Nair D, Badwe R, Head ND, Management G (2015) Elective versus therapeutic neck dissection in node-negative oral cancer. N Engl J Med 373:521–529. https://doi.org/10.1056/NEJMoa1506007
Feng Z, Li JN, Li CZ, Guo CB (2014) Elective neck dissection versus observation in the management of early tongue carcinoma with clinically node-negative neck: a retrospective study of 229 cases. J Craniomaxillofac Surg 42:806–810. https://doi.org/10.1016/j.jcms.2013.11.016
Abu-Ghanem S, Yehuda M, Carmel NN, Leshno M, Abergel A, Gutfeld O, Fliss DM (2016) Elective neck dissection vs observation in early-stage squamous cell carcinoma of the oral tongue with no clinically apparent lymph node metastasis in the neck: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg 142:857–865. https://doi.org/10.1001/jamaoto.2016.1281
Koerdt S, Rockl J, Rommel N, Mucke T, Wolff KD, Kesting MR (2016) Lymph node management in the treatment of oral cancer: analysis of a standardized approach. J Craniomaxillofac Surg 44:1737–1742. https://doi.org/10.1016/j.jcms.2016.08.002
Kesting M (2015). Oral cancer surgery: a visual guide. In Oral Cancer Surgery: A Visual Guide
Tam S, Amit M, Zafereo M, Bell D, Weber RS (2019) Depth of invasion as a predictor of nodal disease and survival in patients with oral tongue squamous cell carcinoma. Head Neck-J Sci Spec 41:177–184. https://doi.org/10.1002/hed.25506
Sakata J, Yamana K, Yoshida R, Matsuoka Y, Kawahara K, Arita H, Nakashima H, Nagata M, Hirosue A, Kawaguchi S, Gohara S, Nagao Y, Hiraki A, Shinohara M, Toya R, Murakami R, Nakayama H (2018) Tumor budding as a novel predictor of occult metastasis in cT2N0 tongue squamous cell carcinoma. Hum Pathol 76:1–8. https://doi.org/10.1016/j.humpath.2017.12.021
Loganathan P, Sayan A, Hsu DWK, Paraneetharan S, Ilankovan V (2017) Squamous cell carcinoma of the anterior tongue: is tumour thickness an indicator for cervical metastasis? Int J Oral Maxillofac Surg 46:407–412. https://doi.org/10.1016/j.ijom.2016.11.003
Lop J, Rigo A, Codina A, de Juan J, Quer M, Leon X (2018) Prognostic significance of extranodal extension in head and neck squamous cell carcinoma cN0 patients with occult metastatic neck nodes. Acta Otorrinolaringol Esp (Engl Ed) 69:156–164. https://doi.org/10.1016/j.otorri.2017.07.002
de Bree R, Takes RP, Castelijns JA, Medina JE, Stoeckli SJ, Mancuso AA, Hunt JL, Rodrigo JP, Triantafyllou A, Teymoortash A, Civantos FJ, Rinaldo A, Pitman KT, Hamoir M, Robbins KT, Silver CE, Hoekstra OS, Ferlito A (2015) Advances in diagnostic modalities to detect occult lymph node metastases in head and neck squamous cell carcinoma. Head Neck 37:1829–1839. https://doi.org/10.1002/hed.23814
Yang L, Liu F, Wu Y, Fang Q, Zhang X, Du W, Zhang X, Chen D, Luo R (2020) Predictive value of occult metastasis and survival significance of metabolic tumor volume determined by PET-CT in cT1-2N0 squamous cell carcinoma of the tongue. Front Oncol 10:542530. https://doi.org/10.3389/fonc.2020.542530
Hutchison IL, Ridout F, Cheung SMY, Shah N, Hardee P, Surwald C, Thiruchelvam J, Cheng L, Mellor TK, Brennan PA, Baldwin AJ, Shaw RJ, Halfpenny W, Danford M, Whitley S, Smith G, Bailey MW, Woodwards B, Patel M, McManners J, Chan CH, Burns A, Praveen P, Camilleri AC, Avery C, Putnam G, Jones K, Webster K, Smith WP, Edge C, McVicar I, Grew N, Hislop S, Kalavrezos N, Martin IC, Hackshaw A (2019) Nationwide randomised trial evaluating elective neck dissection for early stage oral cancer (SEND study) with meta-analysis and concurrent real-world cohort. Br J Cancer 121:827–836. https://doi.org/10.1038/s41416-019-0587-2
de Matos LL, Manfro G, dos Santos RV, Stabenow E, de Mello ES, Alves VAF, Pinto FR, Kulcsar MAV, Brandao LG, Cernea CR (2014) Tumor thickness as a predictive factor of lymph node metastasis and disease recurrence in T1N0 and T2N0 squamous cell carcinoma of the oral tongue. Or Surg or Med or Pa 118:209–217. https://doi.org/10.1016/j.oooo.2014.03.023
Kane SV, Gupta M, Kakade AC, Cruz AD (2006) Depth of invasion is the most significant histological predictor of subclinical cervical lymph node metastasis in early squamous carcinomas of the oral cavity. Ejso-Eur J Surg Onc 32:795–803. https://doi.org/10.1016/j.ejso.2006.05.004
Alvarez Amezaga J, Barbier Herrero L, Pijoan del Barrio JI, Martin Rodriguez JC, Romo Simon L, Genolla Subirats J, Rios Altolaguirre G, de los Rios A, ArteagoitiaCalvo I, LandaLlona S, Arruti Gonzalez JA, Lopez Cedrun J, Santamaria Zuazua J (2007) Diagnostic efficacy of sentinel node biopsy in oral squamous cell carcinoma. Cohort study and meta-analysis. Med Oral Patol Oral Cir Bucal 12:E235-243
Chien CY, Wang CP, Lee LY, Lee SR, Ng SH, Kang CJ, Lin JC, Terng SD, Hua CH, Chen TM, Chen WC, Tsai YT, Tsai CY, Chu YH, Lin CY, Fan KH, Wang HM, Hsieh CH, Yeh CH, Lin CH, Tsao CK, Cheng NM, Fang TJ, Huang SF, Lee LA, Fang KH, Wang YC, Lin WN, Hsin LJ, Yen TC, Wen YW, Liao CT (2023) Indications for elective neck dissection in cT1N0M0 oral cavity cancer according to the AJCC eight edition: a nationwide study. Oral Oncol 140:106366. https://doi.org/10.1016/j.oraloncology.2023.106366
Schilling C, Stoeckli SJ, Haerle SK, Broglie MA, Huber GF, Sorensen JA, Bakholdt V, Krogdahl A, von Buchwald C, Bilde A, Sebbesen LR, Odell E, Gurney B, O’Doherty M, de Bree R, Bloemena E, Flach GB, Villarreal PM, Fresno Forcelledo MF, Junquera Gutierrez LM, Amezaga JA, Barbier L, Santamaria-Zuazua J, Moreira A, Jacome M, Vigili MG, Rahimi S, Tartaglione G, Lawson G, Nollevaux MC, Grandi C, Donner D, Bragantini E, Dequanter D, Lothaire P, Poli T, Silini EM, Sesenna E, Dolivet G, Mastronicola R, Leroux A, Sassoon I, Sloan P, McGurk M (2015) Sentinel European Node Trial (SENT): 3-year results of sentinel node biopsy in oral cancer. Eur J Cancer 51:2777–2784. https://doi.org/10.1016/j.ejca.2015.08.023
Sagheb K, Sagheb K, Rahimi-Nedjat R, Taylor K, Al-Nawas B, Walter C (2016) Sentinel lymph node biopsy in T1/T2 squamous cell carcinomas of the tongue: a prospective study. Oncol Lett 11:600–604. https://doi.org/10.3892/ol.2015.3933
Hyde NC, Prvulovich E, Newman L, Waddington WA, Visvikis D, Ell P (2003) A new approach to pre-treatment assessment of the N0 neck in oral squamous cell carcinoma: the role of sentinel node biopsy and positron emission tomography. Oral Oncol 39:350–360. https://doi.org/10.1016/s1368-8375(02)00121-5
Gallegos-Hernandez JF, Hernandez-Hernandez DM, Flores-Diaz R, Sierra-Santiesteban I, Pichardo-Romero P, Arias-Ceballos H, Minauro-Munoz G, Alvarado-Cabrero I (2005) The number of sentinel nodes identified as prognostic factor in oral epidermoid cancer. Oral Oncol 41:947–952. https://doi.org/10.1016/j.oraloncology.2005.05.010
Ross GL, Soutar DS, Gordon MacDonald D, Shoaib T, Camilleri I, Roberton AG, Sorensen JA, Thomsen J, Grupe P, Alvarez J, Barbier L, Santamaria J, Poli T, Massarelli O, Sesenna E, Kovacs AF, Grunwald F, Barzan L, Sulfaro S, Alberti F (2004) Sentinel node biopsy in head and neck cancer: preliminary results of a multicenter trial. Ann Surg Oncol 11:690–696. https://doi.org/10.1245/ASO.2004.09.001
Alkureishi LW, Burak Z, Alvarez JA, Ballinger J, Bilde A, Britten AJ, Calabrese L, Chiesa C, Chiti A, de Bree R, Gray HW, Hunter K, Kovacs AF, Lassmann M, Leemans CR, Mamelle G, McGurk M, Mortensen J, Poli T, Shoaib T, Sloan P, Sorensen JA, Stoeckli SJ, Thomsen JB, Trifiro G, Werner J, Ross GL (2009) European Association of Nuclear Medicine Oncology C, European Sentinel Node Biopsy Trial C Joint practice guidelines for radionuclide lymphoscintigraphy for sentinel node localization in oral/oropharyngeal squamous cell carcinoma. Ann Surg Oncol 16:3190–3210. https://doi.org/10.1245/s10434-009-0726-8
Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, Forastiere A, Ozsahin EM, Jacobs JR, Jassem J, Ang KK, Lefebvre JL (2005) Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck 27:843–850. https://doi.org/10.1002/hed.20279
Carlton JA, Maxwell AW, Bauer LB, McElroy SM, Layfield LJ, Ahsan H, Agarwal A (2017) Computed tomography detection of extracapsular spread of squamous cell carcinoma of the head and neck in metastatic cervical lymph nodes. Neuroradiol J 30:222–229. https://doi.org/10.1177/1971400917694048
Chai RL, Rath TJ, Johnson JT, Ferris RL, Kubicek GJ, Duvvuri U (2013) Branstetter BFt Accuracy of computed tomography in the prediction of extracapsular spread of lymph node metastases in squamous cell carcinoma of the head and neck. JAMA Otolaryngol Head Neck Surg 139:1187–1194. https://doi.org/10.1001/jamaoto.2013.4491
Haidari S, Obermeier KT, Kraus M, Otto S, Probst FA, Liokatis P (2022) Nodal disease and survival in oral cancer: is occult metastasis a burden factor compared to preoperatively nodal positive neck? Cancers (Basel) 14. https://doi.org/10.3390/cancers14174241
Broglie MA, Haerle SK, Huber GF, Haile SR, Stoeckli SJ (2013) Occult metastases detected by sentinel node biopsy in patients with early oral and oropharyngeal squamous cell carcinomas: impact on survival. Head Neck 35:660–666. https://doi.org/10.1002/hed.23017
Liu JY, Chen CF, Bai CH (2019) Elective neck dissection versus observation in early-stage (cT1/T2N0) oral squamous cell carcinoma. Laryngoscope Investig Otolaryngol 4:554–561. https://doi.org/10.1002/lio2.301
Liu X, Lao X, Liang L, Zhang S, Li K, Liao G, Liang Y (2017) Neck observation versus elective neck dissection in management of clinical T1N0 oral squamous cell carcinoma: a retrospective study of 232 patients. Chin J Cancer Res 29:179–188. https://doi.org/10.21147/j.issn.1000-9604.2017.03.03
Kligerman J, Lima RA, Soares JR, Prado L, Dias FL, Freitas EQ, Olivatto LO (1994) Supraomohyoid neck dissection in the treatment of T1/T2 squamous cell carcinoma of oral cavity. Am J Surg 168:391–394. https://doi.org/10.1016/s0002-9610(05)80082-0
Flach GB, Tenhagen M, de Bree R, Brakenhoff RH, van der Waal I, Bloemena E, Kuik DJ, Castelijns JA, Leemans CR (2013) Outcome of patients with early stage oral cancer managed by an observation strategy towards the N0 neck using ultrasound guided fine needle aspiration cytology: no survival difference as compared to elective neck dissection. Oral Oncol 49:157–164. https://doi.org/10.1016/j.oraloncology.2012.08.006
Nieuwenhuis EJ, Castelijns JA, Pijpers R, van den Brekel MW, Brakenhoff RH, van der Waal I, Snow GB, Leemans CR (2002) Wait-and-see policy for the N0 neck in early-stage oral and oropharyngeal squamous cell carcinoma using ultrasonography-guided cytology: is there a role for identification of the sentinel node? Head Neck 24:282–289. https://doi.org/10.1002/hed.10018
Ho CM, Lam KH, Wei WI, Lau SK, Lam LK (1992) Occult lymph node metastasis in small oral tongue cancers. Head Neck 14:359–363. https://doi.org/10.1002/hed.2880140504
Gane EM, Michaleff ZA, Cottrell MA, McPhail SM, Hatton AL, Panizza BJ, O’Leary SP (2017) Prevalence, incidence, and risk factors for shoulder and neck dysfunction after neck dissection: a systematic review. Eur J Surg Oncol 43:1199–1218. https://doi.org/10.1016/j.ejso.2016.10.026
Dedivitis RA, Guimaraes AV, Pfuetzenreiter EG Jr, Castro MA (2011) Neck dissection complications. Braz J Otorhinolaryngol 77:65–69. https://doi.org/10.1590/s1808-86942011000100011
Schiefke F, Akdemir M, Weber A, Akdemir D, Singer S, Frerich B (2009) Function, postoperative morbidity, and quality of life after cervical sentinel node biopsy and after selective neck dissection. Head Neck 31:503–512. https://doi.org/10.1002/hed.21001
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
AS: conception and design of the study, acquisition, analysis and interpretation of data, drafting the manuscript, and revising it critically for important intellectual content and scientific integrity. MB, TM, RL, AA, and MK: reading and revising the manuscript critically for important intellectual content and scientific integrity. All authors read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was performed in line with the principles of the 1964 Helsinki Declaration and its later amendments. The Ethics Committee of the Friedrich-Alexander University Erlangen-Nuremberg approved the study’s design and methods (Ethic votes: 23–185-Br, 23–186-Br). In accordance with national regulations and institutional regulations, written informed consent was not required from the participating patients.
Conflict of interest
The authors have no financial or non-financial conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Struckmeier, AK., Buchbender, M., Moest, T. et al. Occult metastasis is no burden factor in oral squamous cell carcinoma patients when adhering to a standardized approach in neck dissection. Clin Oral Invest 28, 113 (2024). https://doi.org/10.1007/s00784-024-05514-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00784-024-05514-8