Abstract
Objectives
To examine the effect of lipopolysaccharide (LPS) on cellular senescence induction of human apical papilla cells (hAPCs) and evaluate the potential use of 50 μg/ml ascorbic acid to recover cellular senescence and regenerative functions.
Materials and methods
hAPCs were treated with LPS at 1 and 10 μg/ml either with or without 50 μg/ml ascorbic acid for 48 h. The cellular senescence biomarkers were analyzed by senescence-associated β-galactosidase (SA-β-gal) staining and senescence-related gene expression, p16 and p21. Cell migration, at 12 h and 24 h, was evaluated using a scratch wound assay. Mineralization potential was assessed at 21 days using Alizarin red S staining and dentine sialophosphoprotein (DSPP) and bone sialoprotein (BSP) gene expression.
Results
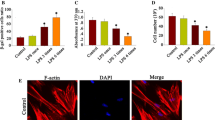
1 μg/ml and 10 μg/ml LPS stimulation for 48 h induced cellular senescence, as shown by remarkable SA-β-gal staining and p16 and p21 gene expression. The percentage of wound closure and mineralized formation was reduced. The co-incubation with ascorbic acid significantly down-regulated the level of SA-β-gal staining. The reduction of senescence-associated gene expressions was observed. Ascorbic acid improved cell migration, mineralized nodule formation, and the expression of DSPP and BSP genes in LPS-treated hAPCs.
Conclusions
LPS significantly promoted cellular senescence on hAPCs and diminished the cell function capacity. Co-presence of ascorbic acid could impede cellular senescence and possibly improve the regenerative capacity of LPS-induced senescent hAPCs in vitro.
Clinical relevance
The data support the in vitro potential benefit of ascorbic acid on cellular senescence recovery of apical papilla cells.



Similar content being viewed by others
References
Torabinejad M, Nosrat A, Verma P, Udochukwu O (2017) Regenerative endodontic treatment or mineral trioxide aggregate apical plug in teeth with necrotic pulps and open apices: a systematic review and meta-analysis. J Endod 43:1806–1820. https://doi.org/10.1016/j.joen.2017.06.029
Chen MH, Chen KL, Chen CA, Tayebaty F, Rosenberg P, Lin L (2012) Responses of immature permanent teeth with infected necrotic pulp tissue and apical periodontitis/abscess to revascularization procedures. Int Endod J 45:294–305. https://doi.org/10.1111/j.1365-2591.2011.01978.x
Nosrat A, Homayounfar N, Oloomi K (2012) Drawbacks and unfavorable outcomes of regenerative endodontic treatments of necrotic immature teeth: a literature review and report of a case. J Endod 38:1428–1434. https://doi.org/10.1016/j.joen.2012.06.025
Becerra P, Ricucci D, Loghin S, Gibbs JL, Lin LM (2014) Histologic study of a human immature permanent premolar with chronic apical abscess after revascularization/revitalization. J Endod 40:133–139. https://doi.org/10.1016/j.joen.2013.07.017
Lin LM, Shimizu E, Gibbs JL, Loghin S, Ricucci D (2014) Histologic and histobacteriologic observations of failed revascularization/revitalization therapy: a case report. J Endod 40:291–295. https://doi.org/10.1016/j.joen.2013.08.024
Verma P, Nosrat A, Kim J, Price J, Wang P, Bair E, Xu H, Fouad A (2017) Effect of residual bacteria on the outcome of pulp regeneration in vivo. J Dent Res 96:100–106. https://doi.org/10.1177/0022034516671499
Huang GTJ, Sonoyama W, Liu Y, Liu H, Wang S, Shi S (2008) The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod 34:645–651. https://doi.org/10.1016/j.joen.2008.03.001
Martin FE, Nadkarni MA, Jacques NA, Hunter N (2002) Quantitative microbiological study of human carious dentine by culture and real-time PCR: association of anaerobes with histopathological changes in chronic pulpitis. J Clin Microbiol 40:1698–1704. https://doi.org/10.1128/jcm.40.5.1698-1704.2002
Narayanan LL, Vaishnavi C (2010) Endodontic microbiology. J Conserv Dent 13:233. https://doi.org/10.4103/2F0972-0707.73386
Cooper PR, Holder MJ, Smith AJ (2014) Inflammation and regeneration in the dentin-pulp complex: a double-edged sword. J Endod 40:S46–S51. https://doi.org/10.1016/j.joen.2014.01.021
Kim JC, Lee YH, Yu MK, Lee NH, Park JD, Bhattarai G, Yi HK (2012) Anti-inflammatory mechanism of PPARγ on LPS-induced pulp cells: role of the ROS removal activity. Arch Oral Biol 57:392–400. https://doi.org/10.1016/j.archoralbio.2011.09.009
Zhang J, Zhang Y, Lv H, Yu Q, Zhou Z, Zhu Q, Wang Z, Cooper PR, Smith AJ, Niu Z (2013) Human stem cells from the apical papilla response to bacterial lipopolysaccharide exposure and anti-inflammatory effects of nuclear factor IC. J Endod 39:1416–1422. https://doi.org/10.1016/j.joen.2013.07.018
Gölz L, Memmert S, Rath-Deschner B, Jäger A, Appel T, Baumgarten G, Götz W, Frede S (2014) LPS from P. gingivalis and hypoxia increases oxidative stress in periodontal ligament fibroblasts and contributes to periodontitis. Mediators Inflamm 2014:1–13. https://doi.org/10.1155/2014/986264
Cheng R, Choudhury D, Liu C, Billet S, Hu T, Bhowmick N (2015) Gingival fibroblasts resist apoptosis in response to oxidative stress in a model of periodontal diseases. Cell Death Discov 1:1–8. https://doi.org/10.1038/cddiscovery.2015.46
Lertchirakarn V, Aguilar P (2017) Effects of lipopolysaccharide on the proliferation and osteogenic differentiation of stem cells from the apical papilla. J Endod 43:1835–1840. https://doi.org/10.1016/j.joen.2017.06.024
Liu J, Du J, Chen X, Yang L, Zhao W, Song M, Wang Z, Wang Y (2019) The effects of mitogen-activated protein kinase signaling pathways on lipopolysaccharide-mediated osteo/odontogenic differentiation of stem cells from the apical papilla. J Endod 45:161–167. https://doi.org/10.1016/j.joen.2018.10.009
Lei S, Liu XM, Liu Y, Bi J, Zhu S, Chen X (2020) Lipopolysaccharide downregulates the osteo-/odontogenic differentiation of stem cells from apical papilla by inducing autophagy. J Endod. https://doi.org/10.1016/j.joen.2020.01.009
Jariyamana N, Chuveera P, Dewi A, Leelapornpisid W, Ittichaicharoen J, Chattipakorn S, Srisuwan T (2021) Effects of N-acetyl cysteine on mitochondrial ROS, mitochondrial dynamics, and inflammation on lipopolysaccharide-treated human apical papilla cells. Clin Oral Investig 25:3919–3928. https://doi.org/10.1007/s00784-020-03721-7
Weekate K, Chuenjitkuntaworn B, Chuveera P, Vaseenon S, Chompu-inwai P, Ittichaicharoen J, Chattipakorn S, Srisuwan T (2021) Alterations of mitochondrial dynamics, inflammation and mineralization potential of LPS-induced human dental pulp cells after exposure to N-acetyl cysteine, Biodentine or ProRoot MTA. Int Endod J 54:951–965. https://doi.org/10.1111/iej.13484
Tan DQ, Suda T (2018) Reactive oxygen species and mitochondrial homeostasis as regulators of stem cell fate and function. Antioxid Redox Signal 29:149–168. https://doi.org/10.1089/ars.2017.7273
Fridlyanskaya I, Alekseenko L, Nikolsky N (2015) Senescence as a general cellular response to stress: a mini-review. Exp Gerontol 72:124–128. https://doi.org/10.1016/j.exger.2015.09.021
Amaya-Montoya M, Pérez-Londoño A, Guatibonza-García V, Vargas-Villanueva A, Mendivil CO (2020) Cellular senescence as a therapeutic target for age-related diseases: a review. Adv Ther 37:1407–1424. https://doi.org/10.1007/s12325-020-01287-0
Campisi J (2005) Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120:513–522. https://doi.org/10.1016/j.cell.2005.02.003
Turinetto V, Vitale E, Giachino C (2016) Senescence in human mesenchymal stem cells: functional changes and implications in stem cell-based therapy. Int J Mol Sci 17:1164. https://doi.org/10.3390/ijms17071164
Yu H, Zhao Y, Luo X, Feng Y, Ren Y, Shang H, He Z, Luo X, Chen S, Wang X (2012) Repeated lipopolysaccharide stimulation induces cellular senescence in BV2 cells. Neuroimmunomodulation 19:131–136. https://doi.org/10.1159/000330254
Kim CO, Huh AJ, Han SH, Kim JM (2012) Analysis of cellular senescence induced by lipopolysaccharide in pulmonary alveolar epithelial cells. Arch Gerontol Geriatr 54:e35–e41. https://doi.org/10.1016/j.archger.2011.07.016
Zhao M, Chen X (2015) Effect of lipopolysaccharides on adipogenic potential and premature senescence of adipocyte progenitors. Am J Physiol - Endocrinol Metab 309:E334–E344. https://doi.org/10.1152/ajpendo.00601.2014
Feng X, Feng G, Xing J, Shen B, Tan W, Huang D, Lu X, Tao T, Zhang J, Li L (2014) Repeated lipopolysaccharide stimulation promotes cellular senescence in human dental pulp stem cells (DPSCs). Cell Tissue Res 356:369–380. https://doi.org/10.1007/s00441-014-1799-7
Aquino-Martinez R, Rowsey JL, Fraser DG, Eckhardt BA, Khosla S, Farr JN, Monroe DG (2020) LPS-induced premature osteocyte senescence: implications in inflammatory alveolar bone loss and periodontal disease pathogenesis. Bone 132:115220. https://doi.org/10.1016/j.bone.2019.115220
Monacelli F, Acquarone E, Giannotti C, Borghi R, Nencioni A (2017) Vitamin C, aging and Alzheimer’s disease. Nutrients 9:670. https://doi.org/10.3390/nu9070670
Staudte H, Güntsch A, Völpel A, Sigusch B (2010) Vitamin C attenuates the cytotoxic effects of Porphyromonas gingivalis on human gingival fibroblasts. Arch Oral Biol 55:40–45. https://doi.org/10.1016/j.archoralbio.2009.11.009
Diomede F, Marconi GD, Guarnieri S, D’Attilio M, Cavalcanti MF, Mariggiò MA, Pizzicannella J, Trubiani O (2019) A novel role of ascorbic acid in anti-inflammatory pathway and ROS generation in HEMA treated dental pulp stem cells. Materials 13:130. https://doi.org/10.3390/ma13010130
Fawzy El-Sayed KM, Nguyen N, Dörfer CE (2020) Ascorbic acid, inflammatory cytokines (IL-1β/TNF-α/IFN-γ), or their combination’s effect on stemness, proliferation, and differentiation of gingival mesenchymal stem/progenitor cells. Stem Cells Int 2020. https://doi.org/10.1155/2020/8897138
Bhandi S, Alkahtani A, Mashyakhy M, Abumelha AS, Albar NHM, Renugalakshmi A, Alkahtany MF, Robaian A, Almeslet AS, Patil VR (2021) Effect of ascorbic acid on differentiation, secretome and stemness of stem cells from human exfoliated deciduous tooth (SHEDs). J Pers Med 11:589. https://doi.org/10.3390/jpm11070589
Ishikawa S, Iwasaki K, Komaki M, Ishikawa I (2004) Role of ascorbic acid in periodontal ligament cell differentiation. J Periodontol 75:709–716. https://doi.org/10.1902/jop.2004.75.5.709
Van Pham P, Tran NY, Phan NL-C, Vu NB, Phan NK (2016) Vitamin C stimulates human gingival stem cell proliferation and expression of pluripotent markers. In Vitro Cell Dev Biol 52:218–227. https://doi.org/10.1007/s11626-015-9963-2
Diederich A, Fründ HJ, Trojanowicz B, Navarrete Santos A, Nguyen AD, Hoang-Vu C, Gernhardt CR (2023) Influence of ascorbic acid as a growth and differentiation factor on dental stem cells used in regenerative endodontic therapies. J Clin Med 12:1196. https://doi.org/10.3390/jcm12031196
Kashino G, Kodama S, Nakayama Y, Suzuki K, Fukase K, Goto M, Watanabe M (2003) Relief of oxidative stress by ascorbic acid delays cellular senescence of normal human and Werner syndrome fibroblast cells. Free Radic Biol Med 35:438–443. https://doi.org/10.1016/S0891-5849(03)00326-5
Kim JE, Jin DH, Lee SD, Hong SW, Shin JS, Lee SK, Jung DJ, Kang JS, Lee WJ (2008) Vitamin C inhibits p53-induced replicative senescence through suppression of ROS production and p38 MAPK activity. Int J Mol Sci 22:651–655. https://doi.org/10.3892/ijmm_00000068
Burger MG, Steinitz A, Geurts J, Pippenger BE, Schaefer DJ, Martin I, Barbero A, Pelttari K (2017) Ascorbic acid attenuates senescence of human osteoarthritic osteoblasts. Int J Mol Sci 18:2517. https://doi.org/10.3390/ijms18122517
Yang M, Teng S, Ma C, Yu Y, Wang P, Yi C (2018) Ascorbic acid inhibits senescence in mesenchymal stem cells through ROS and AKT/mTOR signaling. Cytotechnology 70:1301–1313. https://doi.org/10.1007/s10616-018-0220-x
Yang Y, Wang T, Zhang S, Jia S, Chen H, Duan Y, Wang S, Chen G, Tian W (2021) Vitamin C alleviates the senescence of periodontal ligament stem cells through inhibition of Notch3 during long-term culture. J Cell Physiol 236:1237–1251. https://doi.org/10.1002/jcp.29930
Azman KF, Zakaria R (2019) D-Galactose-induced accelerated aging model: an overview. Biogerontology 20:763–782. https://doi.org/10.1007/s10522-019-09837-y
Zhang D, Yan B, Yu S, Zhang C, Wang B, Wang Y, Wang J, Yuan Z, Zhang L, Pan J (2015) Coenzyme Q10 inhibits the aging of mesenchymal stem cells induced by D-galactose through Akt/mTOR signaling. Oxid Med Cell Longev 2015. https://doi.org/10.1155/2015/867293
Tominaga T, Shimada R, Okada Y, Kawamata T, Kibayashi K (2019) Senescence-associated-β-galactosidase staining following traumatic brain injury in the mouse cerebrum. PLoS One 14:e0213673. https://doi.org/10.1371/journal.pone.0213673
Grada A, Otero-Vinas M, Prieto-Castrillo F, Obagi Z, Falanga V (2017) Research techniques made simple: analysis of collective cell migration using the wound healing assay. J Invest Dermatol 137:e11–e16. https://doi.org/10.1016/j.jid.2016.11.020
Petrino JA, Boda KK, Shambarger S, Bowles WR, McClanahan SB (2010) Challenges in regenerative endodontics: a case series. J Endod 36:536–541. https://doi.org/10.1016/j.joen.2009.10.006
Lenzi R, Trope M (2012) Revitalization procedures in two traumatized incisors with different biological outcomes. J Endod 38:411–414. https://doi.org/10.1016/j.joen.2011.12.003
Liu C, Xiong H, Chen K, Huang Y, Huang Y, Yin X (2016) Long-term exposure to pro-inflammatory cytokines inhibits the osteogenic/dentinogenic differentiation of stem cells from the apical papilla. Int Endod J 49:950–959. https://doi.org/10.1111/iej.12551
Yoo YJ, Oh JH, Lee WC, Woo KM (2016) Regenerative characteristics of apical papilla–derived cells from immature teeth with pulpal and periapical pathosis. J Endod 42:1626–1632. https://doi.org/10.1016/j.joen.2016.08.004
Herranz N, Gil J (2018) Mechanisms and functions of cellular senescence. J Clin Investig 128:1238–1246. https://doi.org/10.1172/JCI95148
Franceschi C, Campisi J (2014) Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 69:S4–S9. https://doi.org/10.1093/gerona/glu057
Sanada F, Taniyama Y, Muratsu J, Otsu R, Shimizu H, Rakugi H, Morishita R (2018) Source of chronic inflammation in aging. Front Cardiovasc Med 5:12. https://doi.org/10.3389/fcvm.2018.00012
Mytych J, Romerowicz-Misielak M, Koziorowski M (2017) Long-term culture with lipopolysaccharide induces dose-dependent cytostatic and cytotoxic effects in THP-1 monocytes. Toxicol in Vitro 42:1–9. https://doi.org/10.1016/j.tiv.2017.03.009
Feng G, Zheng K, Cao T, Zhang J, Lian M, Huang D, Wei C, Gu Z, Feng X (2018) Repeated stimulation by LPS promotes the senescence of DPSCs via TLR4/MyD88-NF-κB-p53/p21 signaling. Cytotechnology 70:1023–1035. https://doi.org/10.1007/s10616-017-0180-6
Mehrazarin S, Oh JE, Chung CL, Chen W, Kim RH, Shi S, Park N-H, Kang MK (2011) Impaired odontogenic differentiation of senescent dental mesenchymal stem cells is associated with loss of Bmi-1 expression. J Endod 37:662–666. https://doi.org/10.1016/j.joen.2011.02.009
Zhang J, An Y, Gao L-N, Zhang Y-J, Jin Y, Chen F-M (2012) The effect of aging on the pluripotential capacity and regenerative potential of human periodontal ligament stem cells. Biomaterials 33:6974–6986. https://doi.org/10.1016/j.biomaterials.2012.06.032
Feng X, Xing J, Feng G, Huang D, Lu X, Liu S, Tan W, Li L, Gu Z (2014) p16INK4A mediates age-related changes in mesenchymal stem cells derived from human dental pulp through the DNA damage and stress response. Mech Ageing Dev 141:46–55. https://doi.org/10.1016/j.mad.2014.09.004
Wu RX, Bi CS, Yu Y, Zhang LL, Chen FM (2015) Age-related decline in the matrix contents and functional properties of human periodontal ligament stem cell sheets. Acta Biomater 22:70–82. https://doi.org/10.1016/j.actbio.2015.04.024
Yi Q, Liu O, Yan F, Lin X, Diao S, Wang L, Jin L, Wang S, Lu Y, Fan Z (2017) Analysis of senescence-related differentiation potentials and gene expression profiles in human dental pulp stem cells. Cells Tissues Organs 203:1–11. https://doi.org/10.1159/000448026
Iezzi I, Cerqueni G, Licini C, Lucarini G, Mattioli Belmonte M (2019) Dental pulp stem cells senescence and regenerative potential relationship. J Cell Physiol 234:7186–7197. https://doi.org/10.1002/jcp.27472
Aguilar P, Mahanonda R, Sa-Ard-Iam N, Lertchirakarn V (2021) Effects of lipopolysaccharide on proliferation, migration and osteogenic differentiation of apical papilla cells from early and late stage of root development. Aust Endod J 47:281–289. https://doi.org/10.1111/aej.12475
Tanaka Y, Sonoda S, Yamaza H, Murata S, Nishida K, Hama S, Kyumoto-Nakamura Y, Uehara N, Nonaka K, Kukita T (2018) Suppression of AKT-mTOR signal pathway enhances osteogenic/dentinogenic capacity of stem cells from apical papilla. Stem Cell Res Ther 9:1–12. https://doi.org/10.1186/s13287-018-1077-9
Tanaka Y, Sonoda S, Yamaza H, Murata S, Nishida K, Kyumoto-Nakamura Y, Uehara N, Nonaka K, Kukita T, Yamaza T (2019) Acetylsalicylic acid treatment and suppressive regulation of AKT accelerate odontogenic differentiation of stem cells from the apical papilla. J Endod 45(591-598):e6. https://doi.org/10.1016/j.joen.2019.01.016
Acknowledgements
The authors would like to thank Adjunct Professor Richard L. Wilson, Faculty Consultant at the Faculty of Dentistry, Chiang Mai University, Chiang Mai, Thailand, for his assistance in the English language editing of the manuscript. The research grant for this project was partially supported by the CMU Mid-career Research Fellowship program and research grants from the Faculty of Dentistry, Chiang Mai University, Chiang Mai, Thailand.
Funding
The research grant for this project was partially supported by the CMU Mid-career Research Fellowship program and research grants from the Faculty of Dentistry, Chiang Mai University, Chiang Mai, Thailand.
Author information
Authors and Affiliations
Contributions
Chananporn Teawcharoensopa: data curation, formal analysis, investigation, software, visualization, writing – original draft preparation. Tanida Srisuwan: conceptualization, funding acquisition, methodology, project administration, resource, supervision, validation, writing – review and editing
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Human Experimental Committee, Faculty of Dentistry, Chiang Mai University (No.5/2021), and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Teawcharoensopa, C., Srisuwan, T. The potential use of ascorbic acid to recover the cellular senescence of lipopolysaccharide-induced human apical papilla cells: an in vitro study. Clin Oral Invest 28, 49 (2024). https://doi.org/10.1007/s00784-023-05455-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00784-023-05455-8