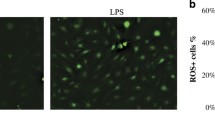
Abstract
Gingival tissue shows progressive changes with aging and an in vitro model of gingival tissue could be useful in understanding age-associated oral diseases. The present study aims to establish a hydrogen peroxide (H2O2) treatment model to induce aging in human gingival epithelial cells. In addition, fisetin, a flavonoid component studied for the anti-aging property is used to examine if it could reverse the induced senescence. Primary human gingival epithelial progenitor (HGEPp) cells were cultured and treated with different concentrations of H2O2. A cell vitality and morphology, senescence-associated beta-galactosidase (SA-β-gal) staining, mRNA and protein expression analysis of known senescence markers p16, p21, and p53, and cell cycle assay were performed. The cells showed dose-dependent changes in vitality and morphology, SA-β-gal staining, relative mRNA and protein expression, and cell cycle assay after H2O2 treatment. Based on these results, 400 μM H2O2 was considered as an optimal concentration to induce senescence. Treatment of senescence-induced cells with fisetin downregulated all the senescence markers used in this study. In conclusion, a senescence model of gingival epithelial cells induced by hydrogen peroxide treatment was established which could be employed to study age-related periodontal diseases.







Similar content being viewed by others
References
Ebersole JL, Graves CL, Gonzalez OA, Dawson D, Morford LA, Huja PE, et al. Aging, inflammation, immunity and periodontal disease. Periodontol. 2016;2000(72):54–75. https://doi.org/10.1111/prd.12135.
Kritsilis M, Rizou S, Koutsoudaki P, Evangelou K, Gorgoulis V, Papadopoulos D. Ageing, cellular senescence and neurodegenerative disease. Int J Mol Sci. 2018;19:297. https://doi.org/10.3390/ijms19102937.
Davalli P, Mitic T, Caporali A, Lauriola A, D’Arca D. ROS, Cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid Med Cell Longev. 2016; Article ID 3565127. https://doi.org/10.1155/2016/3565127
Yosef R, Pilpel N, Papismadov N, Gal H, Ovadya Y, Vadai E, et al. p21 maintains senescent cell viability under persistent DNA damage response by restraining JNK and caspase signaling. EMBO J. 2017;36:2280–95. https://doi.org/10.15252/embj.201695553.
Toussaint O, Medrano EE, von Zglinicki T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp Gerontol. 2000;35:927–45. https://doi.org/10.1016/s0531-5565(00)00180-7.
Suzuki M, Boothman DA. Stress-induced premature senescence (SIPS). J Radiat Res. 2008;49:105–12. https://doi.org/10.1269/jrr.07081.
Ott C, Jung T, Grune T, Höhn A. SIPS as a model to study age-related changes in proteolysis and aggregate formation. Mech Ageing Dev. 2018;170:72–81. https://doi.org/10.1016/j.mad.2017.07.007.
Chen Q, Ames BN. Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proc Natl Acad Sci. 1994;91:4130–4. https://doi.org/10.1073/pnas.91.10.4130.
Ohguro N, Fukuda M, Sasabe T, Tano Y. Concentration dependent effects of hydrogen peroxide on lens epithelial cells. Br J Ophthalmol. 1999;83:1064–8. https://doi.org/10.1136/bjo.83.9.1064.
Yoshizaki K, Katakura Y, Fujiki T, Teruya K, Shirahata S. Hydrogen peroxide triggers cellular senescence programs: possible application to tumor suppression. In: Yagasaki K, Miura Y, Hatori M, Nomura Y, editors. Animal cell technology: basic and applied aspects, vol. 13. Springer; 2003.
Duan J, Duan J, Zhang Z, Tong T. Irreversible cellular senescence induced by prolonged exposure to H2O2 involves DNA-damage-and-repair genes and telomere shortening. Int J Biochem Cell Biol. 2005;37:1407–20. https://doi.org/10.1016/j.biocel.2005.01.010.
Yousefzadeh MJ, Zhu Y, McGowan SJ, Angelini L, Fuhrmann-Stroissnigg H, Xu M, et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018;36:18–28. https://doi.org/10.1016/j.ebiom.2018.09.015.
Maher P. How fisetin reduces the impact of age and disease on CNS function. Front Biosci. 2015;7:58–82. https://doi.org/10.2741/425.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–8. https://doi.org/10.1006/meth.2001.1262.
Burdon RH, Alliangana D, Gill V. Hydrogen peroxide and the proliferation of Bhk-21 cells. Free Radic Res. 1995;23:471–86. https://doi.org/10.3109/10715769509065268.
Wiese AG, Pacifici RE, Davies KJA. Transient adaptation to oxidative stress in mammalian cells. Arch Biochem Biophys. 1995;318:231–40. https://doi.org/10.1006/abbi.1995.1225.
Zdanov S. Establishment of H2O2-induced premature senescence in human fibroblasts concomitant with increased cellular production of H2O2. Ann N Y Acad Sci. 2006;1067:210–6. https://doi.org/10.1196/annals.1354.025.
Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci. 1995;92:9363–7. https://doi.org/10.1073/pnas.92.20.9363.
Tominaga T, Shimada R, Okada Y, Kawamata T, Kibayashi K. Senescence-associated-β-galactosidase staining following traumatic brain injury in the mouse cerebrum. PLoS ONE. 2019;14: e0213673. https://doi.org/10.1371/journal.pone.0213673.
Chen QM, Tu VC, Catania J, Burton M, Toussaint O, Dilley T. Involvement of Rb family proteins, focal adhesion proteins and protein synthesis in senescent morphogenesis induced by hydrogen peroxide. J Cell Sci. 2000;113:4087–97.
Capparelli C, Chiavarina B, Whitaker-Menezes D, Pestell TG, Pestell RG, Hulit J, et al. CDK inhibitors (p16/p19/p21) induce senescence and autophagy in cancer-associated fibroblasts, “fueling” tumor growth via paracrine interactions, without an increase in neo-angiogenesis. Cell Cycle. 2012;11:3599–610. https://doi.org/10.4161/cc.21884.
Rufini A, Tucci P, Celardo I, Melino G. Senescence and aging: the critical roles of p53. Oncogene. 2013;32:5129–43. https://doi.org/10.1038/onc.2012.640.
Kuliman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24:2463–79. https://doi.org/10.1101/gad.1971610.
Mao Z, Ke Z, Gorbunova V, Seluanov A. Replicatively senescent cells are arrested in G1 and G2 phases. Aging. 2012;4:431–5. https://doi.org/10.18632/aging.100467.
Shapiro GI, Edwards CD, Ewen ME, Rollins BJ. p16INK4A participates in a G1 arrest checkpoint in response to DNA damage. Mol Cell Biol. 1998;18:378–87. https://doi.org/10.1128/mcb.18.1.378.
Gire V, Dulić V. Senescence from G2 arrest, revisited. Cell Cycle. 2015;14:297–304. https://doi.org/10.1080/15384101.2014.1000134.
Johmura Y, Shimada M, Misaki T, Naiki-Ito A, Miyoshi H, Motoyama N, et al. Necessary and sufficient role for a mitosis skip in senescence induction. Mol Cell. 2014;55:73–84. https://doi.org/10.1016/j.molcel.2014.05.003.
Dash BC, El-Deiry WS. Phosphorylation of p21 in G2/M promotes cyclin B-Cdc2 kinase activity. Mol Cell Biol. 2005;25:3364–87.
Herbig U, Sedivy JM. Regulation of growth arrest in senescence: telomere damage is not the end of the story. Mech Ageing Dev. 2006;127:16–24. https://doi.org/10.1016/j.mad.2005.09.002.
Naeimi AF, Alizadeh M. Antioxidant properties of the flavonoid fisetin: an updated review of in vivo and in vitro studies. Trends Food Sci Technol. 2017;70:34–44. https://doi.org/10.1016/j.tifs.2017.10.003.
Seo S-H, Jeong G-S. Fisetin inhibits TNF-α-induced inflammatory action and hydrogen peroxide-induced oxidative damage in human keratinocyte HaCaT cells through PI3K/AKT/Nrf-2-mediated heme oxygenase-1 expression. Int Immunopharmacol. 2015;29:246–53. https://doi.org/10.1016/j.intimp.2015.11.014.
Sun X, Ma X, Li Q, Yang Y, Xu X, Sun J, et al. Anti-cancer effects of fisetin on mammary carcinoma cells via regulation of the PI3K/Akt/mTOR pathway: Invitro and invivo studies. Int J Mol Med. 2018;42:811–20. https://doi.org/10.3892/ijmm.2018.3654.
Sun Q, Zhang W, Zhong W, Sun X, Zhou Z. Dietary fisetin supplementation protects against alcohol-induced liver injury in mice. Alcohol Clin Exp Res. 2016;40:2076–84. https://doi.org/10.1111/acer.13172.
Resende FA, Vilegas W, dos Santos LC, Varanda EA. Mutagenicity of flavonoids assayed by bacterial reverse mutation (Ames) test. Molecules. 2012;17:5255–68. https://doi.org/10.3390/molecules17055255.
Imran M, Saeed F, Gilani SA, Shariati MA, Imran A, Afzaal M, et al. Fisetin: an anticancer perspective. Food Sci Nutr. 2020;9:3–16. https://doi.org/10.1002/fsn3.1872.
Funding
Grant was received to conduct this study.
Author information
Authors and Affiliations
Contributions
YF conceptualized the idea. YF, AT, KM and MF designed the study. SG, AT, KY and YK conducted the experiments. SG, DP, YA and YF analyzed and interpreted the data. SG wrote the initial draft of the manuscript. YA and YF contributed in the critical revision of the manuscript. All authors agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Giri, S., Takada, A., Paudel, D. et al. An in vitro senescence model of gingival epithelial cell induced by hydrogen peroxide treatment. Odontology 110, 44–53 (2022). https://doi.org/10.1007/s10266-021-00630-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-021-00630-3