Abstract
Objective
To provide a critical overview of the effect of various orthodontic and/or dentofacial orthopedic interventions on three-dimensional volumetric changes in the upper airway.
Materials and methods
Four databases were searched for clinical studies concerning 3D volumetric assessments based on CBCT before and after orthodontics interventions. The quality of the studies was assessed using the quality assessment tool of the National Heart, Lung and Blood Institute. After the use of inclusion and exclusion criteria, the pre-and post-treatment volumes were used to visualize the effect of various orthodontics interventions.
Results
A total of 48 studies were included in this review and none of which were RCTs. The quality of all included studies was assessed as medium. Overall, there is a tendency for an increase in airway volumes after various orthodontic interventions, except for studies concerning extraction therapy with fixed appliances in adults, in which both increases and decreases in airway volumes have been reported.
Conclusion
Orthodontic treatment by growth modification and non-extraction therapy with fixed appliances, regardless of the malocclusion, generally showed positive effects on the airway volume. Orthodontic treatment in combination with extractions does not provide an unambiguous insight. A consensus on the methodology of the airway measurement and nomenclature is urgently needed in order to gain insight into the effect of different interventions on three-dimensional airway changes.
Clinical relevance
Various orthodontic treatments do not negatively influence the upper airway volume. However, extraction therapy in adults should be chosen with caution, especially in subjects belonging to a group susceptible to airway obstruction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The primary objective of orthodontic treatment is to establish an optimal dental and/or skeletal relationship in harmony with the morphology and function of the soft tissues in the oro-maxillofacial region. In addition, facilitating the development and functional demands of the airway is an important objective, especially in patients susceptible to airway obstruction or sleep apnea. Already in 1907, at the onset of orthodontics being established as a dental specialty, Angle postulated that children with a retrognathic mandible could have a smaller airway dimension. Recent studies showed that in patients with obstructive sleep apnea the underlying skeletal deformities are indeed related to a relatively restricted upper airway dimension [1,2,3,4,5,6].
Traditionally, airway dimensions were assessed using lateral cephalograms [7]. However, cephalometric measurements have severe limitations in accessing the airway, as only changes in the sagittal and vertical dimensions can be observed. Thereby neglecting the volumetric- and transversal dimensions of the airway. Moreover, 2D cephalometric and 3D volumetric measurements of the airway on CBCT [8, 9] are not a correlated. Accurate determination of the airway dimensions on a lateral cephalogram is difficult because of a large variation in 2D airway landmarks. As a better alternative, a CT, CBCT, or MRI scan could be used to assess the airway in all three dimensions. However, the costs of a CT or MRI scan are high, and the radiation dose of a multi-slice CT is much higher compared to a CBCT scan [10]. Also, in a CT scan, patients are usually in the supine position, resulting in an effect of gravity on soft tissues around the airway and therewith an error in the volume measurement on the scan will occur [11]. CBCT scans, in comparison, have much shorter image acquisition times, reducing the chance of movement of the patient during the acquisition, and providing the opportunity to perform measurements in volume, cross-sectional area, choke point, width, length, and anterior posterior dimensions of the airway. A recent systematic review concluded that airway measurements on CBCT scans have moderate to excellent reliability[12].
In the current literature, the effect of orthodontic treatment on volumetric changes in the upper airway provides multiple outcomes. Previous reviews on volumetric changes in the airway focused on one type of treatment intervention, e.g. extraction therapy with fixed appliances [13], maxillary expansion [14], and treatment of Class II malocclusion with functional appliances [15]. Due to the differences in intervention types and high heterogeneity in the definition of the airway and/or its segments, it is not possible to make relevant comparisons of the findings between different interventions or to provide a valid interpretation of the outcomes from these reviews. Moreover, no previous reviews have investigated the effect of orthodontic treatment of Class III malocclusion on the airway.
Here we aim to provide a systematic analysis of the effect of different orthodontic interventions, including transversal and sagittal growth modifications, and extraction and non-extraction therapies with fixed appliances, on 3D volumetric changes of the upper airway using a standardized nomenclature with reliable anatomical landmarks to determine the borders of the airway on CBCT scans.
Methods
Protocol and registration
The protocol is registered in the International Platform of Registered Systematic Review and Meta-analysis Protocols INPLASY (https://inplasy.com/) under number INPLASY202240017.
(DOI number https://doi.org/10.37766/inplasy2022.4.0017). The PRISMA 2020 checklist was used for reporting this systematic review [16, 17].
Eligibility criteria
The research question was formulated by means of the Population, Intervention, Comparison, Outcome, and Study Design (PICOS) framework. The research question was: does the volume of the upper airway change after orthodontic intervention?
-
P: growing subjects, adults
-
I: orthodontic treatment, dentofacial orthopedics, extractions
-
C: untreated subjects and/or subjects having fixed appliances treatment with non-extractions
-
O: volumetric changes of the upper airway measured on CBCT scans
-
S: randomized controlled trials (RCTs), controlled clinical trials, prospective cohort studies, observational studies, intervention studies
Inclusion criteria were: healthy human subjects aged 7 years and older, of any sex and with any types of orthodontic malocclusion; Subjects have had one or more of the following interventions: full orthodontic treatment with fixed appliances, or aligners with or without extraction of premolars, transversal growth modification with expansion appliances, sagittal growth modification of Class II or Class III malocclusions with functional appliances; Randomized controlled trials (RCT's), controlled clinical trials, prospective cohort studies, observational studies, intervention studies with orthodontics as intervention; Treatment group > 10 participants; CBCT acquisition with the patient positioned upright, and pre-and post-treatment 3D volumetric assessments of the airway available with clear definition or illustration of the airway.
Exclusion criteria: subjects with syndromes, cleft lip and/or palate, systemic diseases relating to orofacial growth, or OSAS and/or other airway diseases.
Information sources and search strategy
A search was conducted in the electronic databases of PubMed, EMBASE, Web of Science, and the Cochrane Library. The 1st of April 2023 was marked as the end date of the search. The search strategy for each database was as follows:
-
PubMed:
-
(‘orthodontics’[Mesh] OR orthodont*[tiab] OR dentofacial*[tiab])
-
AND
-
(‘respiratory System’[Mesh] OR respirat*[tiab] OR airway*[tiab] OR pharynx*[tiab] OR nasopharynx*[tiab] OR oropharynx*[tiab] OR hypopharynx*[tiab])
-
EMBASE:
-
('orthodontics'/exp OR (orthodont* OR dentofacial*):ab,ti,kw)
-
AND
-
('respiratory system'/exp OR (respirat* OR airway* OR pharynx* OR nasopharynx* OR oropharynx* OR hypopharynx*):ab,ti,kw)
-
Web of Science:
-
TS = (orthodont* OR dentofacial*)
-
AND
-
TS = (respirat* OR airway* OR pharynx* OR nasopharynx* OR oropharynx* OR hypopharynx*)
-
Cochrane:
-
(orthodont* OR dentofacial*)
-
AND
-
(respirat* OR airway* OR pharynx* OR nasopharynx* OR oropharynx* OR hypopharynx*)
All studies were retrieved with no restrictions for language or article status. Eventually, the search was updated until 1st April 2023. Furthermore, manual screening of the reference lists of the studies included in the systematic review was performed. Grey literature was not searched.
Study selection
Two authors (RS and AS), working independently, reviewed titles and abstracts (unblinded) on all the exclusion criteria. When this was insufficient the full text was screened only on exclusion criteria. The full text of the remaining articles was independently screened by the same two authors on the inclusion criteria. To be included all inclusion criteria must be met. In case of disagreement, a consensus was reached by discussion, or the third reviewer (YR) was consulted if needed. All studies were exported to an open-source reference manager software Zotero (Center for History and New Media version 6.0.19).
Data items and data collection process
A data extraction form was developed and piloted in Covidence. Two reviewers (RS, AS) extracted the data from the included studies. Data were extracted for volumetric measurements before and after treatment intervention. If disagreement existed, it was resolved through discussion with the third reviewer (YR).
Summary measures
Volumetric changes of the total upper airway and of its individual segments, as measured on CBCT scans were selected as the main (primary) outcome measure. Mean volumetric changes in mm3 were used and if available, the standard deviation (SD) from the original publication.
Comparisons of the effect on the airway of different orthodontic/orthopedic intervention categories were selected as the additional/secondary outcome.
Anatomical landmarks, borders, and reference planes of the airway
Considering the large heterogeneity and inconsistency in the definition of the upper airway and its segments, we defined for data analysis, five cross-sectional planes (two frontal and three axial). These are based on five soft and hard tissue anatomical landmarks on the mid-sagittal plane (Fig. 1 and Table 1).
Definition of the upper airway and its segments used in this systematic review for data analysis of the included studies. The purple line indicates the most superior border of the Airway. The Red line indicates the lower border of the Nasopharynx and the upper border of the oropharynx. The green line indicates the lower border of the Oropharynx and upper border of the hypopharynx, and the orange line indicates the most inferior border of the hypopharynx. 1 = most inferior point of the floor of the sphenoid sinus, 2 = Posterior Nasal Spine, 3 = anterior superior part of C2, 4 = posterior inferior part of the C2, 5 = superior anterior part of C4, 6 = superior part of the epiglottis, 7 = anterior inferior part of C4, 8 = bottom of the epiglottis
Reference fields for the upper airway and its segments
Data retrieved from the original studies were standardized following a previously published protocol, based on the concept of ‘reference fields’ that accommodates a pre-defined, limited range of variations in the reference plane [18]. Briefly, the anatomical landmarks and reference planes used in the original studies were compared to the proposed reference fields that are illustrated in Fig. 2.
Reference fields for the upper airway and its segments. Each color block represents a ‘reference field’ that accommodates a pre-defined, limited range of variations of the respective reference plane (line in the same color). The yellow triangle indicates variations of the anterior borders accepted for data analysis in this review (B), the purple box indicates variations of the superior border of the nasopharynx (A), the red box indicates variations of the superior borders of the oropharynx (C), and the green box indicates variations of the inferior borders of the oropharynx (D)
Volumetric data inclusion and interpretation using the reference fields
The following protocol was applied on pre-, and post-treatment volumetric data extracted from the included studies using the reference fields described above.
-
1)
Data inclusion without additional validation: original data were included directly when the definition of the airway and its segments concurs with the proposed reference planes (Table 1, Fig. 1).
-
2)
Data inclusion after additional validation (in italics in Table 3): original data were included when the definition of the airway and its segments falls within the proposed reference fields (Fig. 2).
-
3)
Data exclusion: original data were excluded when the definition of the airway and its segments falls outside the proposed reference fields (Fig. 2).
In the case of multiple post-treatment follow-ups, the longest follow-up results were used.
Risk of bias in individual studies
The quality of the included studies was assessed according to the quality assessment tool of the National Heart, Lung, and Blood Institute (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools). Depending on the type of study, the quality assessment tool for “Case-control Studies” or, if applicable, for “Before-After (Pre-Post) Studies with no control group” was used. Rating of a study was done according to a questionnaire of twelve questions, answered by ‘yes’ or ‘no’, whereas ‘yes’ scores one point and ‘no’ scores no point. A maximum of 12 points could be obtained. A score of 1–4 qualified as poor, 5–9 as fair, and 10–12 as good. Two reviewers performed the rating independently (RS, AS).
Disagreements were discussed and solved with a third author (YR).
Additional analysis
A bar graph was generated to visualize the relative changes in the airway and its segments resulting from different types of orthodontic interventions.
Planned methods of analysis
First, heterogeneity between the studies was assessed based on population, age, treatment, and follow-up period. Due to a large heterogeneity between studies, a quantitative analysis was not possible, and a descriptive synthesis was conducted.
Results
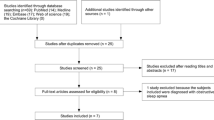
Study selection (Fig. 3)
A total of 7069 articles were retrieved after the first search with one additional hit after a hand search or from citations. Figure 3 illustrates the PRISMA 2020 Flow Diagram and a detailed overview of the selection process. After the removal of the duplicates, 4419 articles remained for further screening of titles and abstracts. A total of 88 articles were eligible for the full-text assessment of the inclusion criteria. Out of the 88 articles, 4 full texts were not retrievable. Of these, 35 studies were excluded due to predetermined exclusion criteria. Finally, after the additional hand search, 50 studies met the inclusion criteria for this systematic review.
Study characteristics (Table 2)
In Table 2 the characteristics of the total of 48 included studies are presented. From these 48 studies, 71 treatment groups (N) were identified and divided into the following three intervention categories:
-
1.
Non-extraction growth modification (N = 46);
-
1.1 Maxillary transversal growth modification (N = 27)
-
1.2 Sagittal growth modification of Angle Class III malocclusion (N = 6)
-
1.3 Sagittal growth modification of Angle Class II malocclusion (N = 13)
-
-
2.
Non-extraction therapy with fixed appliances or aligners without prior dentofacial orthopedic therapy (N = 14).
-
3.
Extraction therapy with fixed appliances or aligners without prior dentofacial orthopedic therapy (N = 11).
The studies on growth modification involved only growing patients (1.1, 1.2, 1.3), while those using fixed appliances or aligners involved both growing and adult subjects (2 and 3). Follow-up in the studies varied from 1 month up to 42 months, with 24 months being the most frequent follow-up.
Risk of bias within studies (Table 2)
In 48 included studies, only 16 reported a power-analysis (or post-hoc analysis) to determine the minimal number of subjects needed. No randomized controlled trials could be identified. Except for one unknown [29] and two multi-center studies [26, 40], all included studies were single-center based. Eight studies had a prospective and 40 a retrospective study design. Four studies had an untreated control group with both pre-and post-treatment CBCT scans [21, 25, 43, 52]. Three studies included an untreated control group, with only post-treatment CBCT scans available [44, 49, 54]. In five studies on growth modification, age-matched subjects treated with ‘non-extraction fixed appliances’ served as a control [28, 31, 32, 48, 53]. In six other studies, subjects with ‘extraction fixed appliances’ were compared to subjects with ‘non-extraction fixed appliances’ [56,57,58,59,60,61,62].
Three studies were rated as ‘good’ (score 10), and the other included studies were qualified as ‘medium risk of bias’. Forty-four studies scored between 5 to 9, indicating ‘fair quality’. No studies scored under 5 points (poor quality).
Main outcomes
Airway volumetric changes in relation to different interventions
Airway volumetric changes in mm3 after different types of interventions are presented in Table 3. Among the three airway segments, oropharynx volumes were reported in all studies except five [20, 33, 38, 39, 42] on maxillary transversal expansion, one on Class III growth modifications [45], one on fixed appliances treatment [61] and one on fixed appliances with extractions [64]. Nasopharynx volumes were reported in more than half of the studies on maxillary transversal expansion but in less than half of the other treatment groups. Only five studies reported the volumes on the hypopharynx airway [22, 23, 28, 46, 49].
An overall increase in the airway volume was shown in studies with growth modification and fixed appliances treatment without extraction, regardless of the pre-treatment malocclusion (Table 3 Sections 1.1, 1.2, 1.3, and “Methods”).
Results for fixed appliances therapy with extraction were less consistent, with both increase and decrease of volumes in the airway being reported, though the change was significant only in one study (p < 0.05) [60]. This inconsistency can be related to the age of the study subjects, as a decrease in the volume of the airway was observed only in adult patients[26, 55, 58, 60] while an increase was observed mostly in growing adolescents [59, 60].
Additional outcomes
In Supplementary files 1 to 5 bar graphs are presented to illustrate the percentages of post-treatment volumetric changes in relation to the respective pre-treatment level. The study of Iwasaki et al. reported an exceeding post-treatment volumetric increase of 219%, attributed to a very long follow-up (42 months), and was therefore excluded from the bar [48]. Patterns can be recognized for different treatment modalities. Volumes of the airway in studies with dentofacial-orthopedic growth modification showed almost all increases, up to 60% of the pre-treatment levels, regardless of the power of the study or the type of interventions. The increases were observed most frequently in the oropharynx (Supp. 1, 2 and 3). Treatment with fixed appliances showed distinguishable features in the oropharynx airway between extraction and non-extraction therapies. An overall increase of the volume was observed, up to 55% of the pre-treatment level after non-extraction therapy (Supp. 4). Extraction therapy, on the other hand, resulted in changes in both positive and negative directions, though to a lesser degree compared with non-extraction therapy (Supp. 5).
Discussion
Summary of evidence
Orthodontic and dentofacial orthopedic treatment modifies the position of the skeletal, dental, and soft tissues within the maxillofacial complex. Therewith the soft tissues surrounding the upper airway may adapt to a new position, resulting in volumetric changes in the airway. The present review included all eligible studies on 3D volumetric changes in the upper airway after orthodontic and/or dentofacial orthopedic interventions. A meta-analysis could not be performed due to the high level of heterogeneity in the volumetric data, resulting from large variations of the defined anatomical borders of the airway.
Results from the present review did not show any evidence of a negative impact of orthodontic interventions on airway volumes, during the observation periods. The only exception might be extraction therapy (of premolars), in which a tendency of volumetric decrease in the airway was observed in adult subjects [57, 58]. However, changes in the airway were small and statistically not significant and amounted to a maximum of—8% of the original values. Orthodontic extraction therapy is often related to the shortening of the anterior-posterior arch length and retraction of the anterior teeth. These changes may lead to the backward movement of the tongue that compresses the soft palate and narrows the oropharynx airway. However, evidence is lacking to support such a causal effect. Growing subjects may accommodate broader indications for extraction therapy, without normal growth of the airway volume being impeded during the treatment period. In comparison, studies on non-extraction therapy almost all showed a volumetric increase in the airway up to 55% of the pre-treatment level, with the largest changes seen in subjects between 9 to 12.0 years of age [28, 32].
Among the three types of growth modification therapy, the most notable change was in patients treated with maxillary expansion. In which the volumes increased in all three airway segments. In this group, the subjects were relatively young, with a range of the average ages between 7.9 to 14.7 years, except for one non-controlled study with a small sample (N = 13) of young adults aged 19.6 years and a follow-up of only 3 months, with a quality score of 5 [36]. An average of 13% volumetric increase was found in the airway across all included studies on maxillary expansion. This appears comparable with an average of 10% in studies on surgically assisted maxillary expansion in adults reported in a previous review [18].
In 7 out of 13 studies on growth modifications in subjects with Class II malocclusion, the post-treatment airway volumes were significantly higher than the pre-treatment level and/or the age-matched controls especially in the oropharynx. Demonstrating an additional gain from the intervention. These results are in line with a recent review, reporting weak evidence for a volumetric increase in the upper airway based on 5 studies on treatment with functional appliances in patients with Class II malocclusion [15].
Growth modifications in subjects with a Class III malocclusion showed a volumetric increase in different airway segments. All studies in this category had a reasonable quality, although two studies had no control group which means the effects of normal growth and therapy cannot be separated. In all included studies, except for the study of Liu et al. [46],a protraction force was applied to the maxilla to enhance the forward and downward growth of the maxilla. Out of 6 study groups, five demonstrated a significant increase in the volume in at least one airway segment. An average of 18% volumetric increase in the airway across all included studies in this category of intervention, is higher than that of 14% in patients undergoing a single jaw Le-Fort I advancement reported in a previous review [18], which may be attributed to a combined effect of favorable treatment reactions and normal growth in the airway.
Though some patterns could be recognized in the outcome from the present review, one has to bear in mind that volumetric changes in the upper airway are influenced by multiple factors, such as initial indications (crowding or retraction) for extraction [13], retraction of the upper- and lower incisors [57, 64] and dental alignment of crowding [60]. It is, therefore, not possible to draw a firm conclusion concerning the effect of one specific type of intervention.
Limitations
One limitation of the current review is the wide range of follow-up lengths between the included studies. Obviously, studies with longer follow-up periods will cover a greater span of normal growth, which may result in both larger absolute volumetric measurements and relative percentual changes. Another limitation is that no randomized controlled trials could be included, even though the quality of all included studies was assessed as medium. Additionally, the absence of an untreated control group in many of the included studies is a matter of discussion, as it makes it challenging to distinguish the genuine treatment effect from normal growth.
Conclusions and Recommendations for future research
Taking into account the acknowledged limitations, the present review concludes that orthodontic treatment, regardless of the type of intervention, malocclusion, or patient age, did not yield evidence for changes in upper airway volume whether positive or negative.
A joint endeavor in the dental community to establish a consensus on airway measurement methodology and terminology, including the various segments, will greatly enhance the quality and comparability of studies on volumetric changes in the airway. Future studies may focus on extraction therapy in adults, particularly those susceptible to airway obstruction, in order to identify potential risk factors that impede airway growth. Other clinically relevant parameters such as the average cross-sectional surface areas and choke points (minimal cross-sectional areas) in airway evaluation, in addition to volumetric measurements in cubic millimeters, may also be considered.
References
Lowe AA, Santamaria JD, Fleetham JA, Price C (1986) Facial morphology and obstructive sleep apnea. Am J Orthod Dentofac Orthop 90:484–491. https://doi.org/10.1016/0889-5406(86)90108-3
Hui DSC, Ko FWS, Chu ASY, Fok JPC, Chan MCH, Li TST, Choy DKL, Lai CKW, Ahuja A, Ching ASC (2003) Cephalometric assessment of craniofacial morphology in Chinese patients with obstructive sleep apnoea. Respir Med 97:640–646. https://doi.org/10.1053/rmed.2003.1494
Deng J, Gao X (2012) A case–control study of craniofacial features of children with obstructed sleep apnea. Sleep Breath 16:1219–1227. https://doi.org/10.1007/s11325-011-0636-4
Katyal V, Pamula Y, Martin AJ, Daynes CN, Kennedy JD, Sampson WJ (2013) Craniofacial and upper airway morphology in pediatric sleep-disordered breathing: Systematic review and meta-analysis. Am J Orthod Dentofac Orthop 143:20-30.e3. https://doi.org/10.1016/j.ajodo.2012.08.021
Roedig JJ, Phillips BA, Morford LA, Van Sickels JE, Falcao-Alencar G, Fardo DW, Hartsfield JK, Ding X, Kluemper GT (2014) Comparison of BMI, AHI, and Apolipoprotein E ε4 (APOE-ε4) Alleles among Sleep Apnea Patients with Different Skeletal Classifications. J Clin Sleep Med 10:397–402. https://doi.org/10.5664/jcsm.3614
Zicari AM, Duse M, Occasi F, Luzzi V, Ortolani E, Bardanzellu F, Bertin S, Polimeni A (2014) Cephalometric Pattern and Nasal Patency in Children with Primary Snoring: The Evidence of a Direct Correlation. PLoS ONE 9:e111675. https://doi.org/10.1371/journal.pone.0111675
Kinzinger G, Czapka K, Ludwig B, Glasl B, Gross U, Lisson J (2011) Effects of fixed appliances in correcting Angle Class II on the depth of the posterior airway space: FMA vs. Herbst appliance—a retrospective cephalometric study. J Orofac Orthop 72:301–320. https://doi.org/10.1007/s00056-011-0035-2
Abe-Nickler MD, Portner S, Sieg P, Hakim SG (2017) No correlation between two-dimensional measurements and three-dimensional configuration of the pharyngeal upper airway space in cone-beam computed tomography. J Craniomaxillofac Surg 45:371–376. https://doi.org/10.1016/j.jcms.2017.01.004
Aboudara C, Nielsen I, Huang JC, Maki K, Miller AJ, Hatcher D (2009) Comparison of airway space with conventional lateral headfilms and 3-dimensional reconstruction from cone-beam computed tomography. Am J Orthod Dentofacial Orthop 135:468–479. https://doi.org/10.1016/j.ajodo.2007.04.043
Roberts JA, Drage NA, Davies J, Thomas DW (2009) Effective dose from cone beam CT examinations in dentistry. BJR 82:35–40. https://doi.org/10.1259/bjr/31419627
Sutthiprapaporn P, Tanimoto K, Ohtsuka M, Nagasaki T, Iida Y, Katsumata A (2008) Positional changes of oropharyngeal structures due to gravity in the upright and supine positions. Dentomaxillofac Rad 37:130–136. https://doi.org/10.1259/dmfr/31005700
Zimmerman JN, Lee J, Pliska BT (2016) Reliability of upper pharyngeal airway assessment using dental CBCT: a systematic review. EORTHO cjw079. https://doi.org/10.1093/ejo/cjw079
Hu Z, Yin X, Liao J, Zhou C, Yang Z, Zou S (2015) The effect of teeth extraction for orthodontic treatment on the upper airway: a systematic review. Sleep Breath 19:441–451. https://doi.org/10.1007/s11325-015-1122-1
Niu X, Di Carlo G, Cornelis MA, Cattaneo PM (2020) Three-dimensional analyses of short- and long-term effects of rapid maxillary expansion on nasal cavity and upper airway: A systematic review and meta-analysis. Orthod Craniofac Res 23:250–276. https://doi.org/10.1111/ocr.12378
Li J, Ge X, Guan H, Zhang S, Qiao X, Chang W, Ma W (2021) Three-dimensional changes of the upper airway in patients with Class II malocclusion treated with functional appliances: a systematic review and meta-analysis. Eur J Orthod 43:415–423. https://doi.org/10.1093/ejo/cjaa080
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ n71. https://doi.org/10.1136/bmj.n71
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE (2021) PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ n160. https://doi.org/10.1136/bmj.n160
Steegman R, Hogeveen F, Schoeman A, Ren Y (2022) Cone beam computed tomography volumetric airway changes after orthognathic surgery: a systematic review. Int J Oral Maxillofac Surg S0901502722002260. https://doi.org/10.1016/j.ijom.2022.05.013
Kavand G, Lagravere M, Kula K, Stewart K, Ghoneima A (2019) Retrospective CBCT analysis of airway volume changes after bone-borne vs tooth-borne rapid maxillary expansion. Angle Orthod 89:566–574. https://doi.org/10.2319/070818-507.1
Lanteri V, Farronato M, Ugolini A, Cossellu G, Gaffuri F, Parisi FMR, Cavagnetto D, Abate A, Maspero C (2020) Volumetric Changes in the Upper Airways after Rapid and Slow Maxillary Expansion in Growing Patients: A Case-Control Study. Materials 13:2239. https://doi.org/10.3390/ma13102239
Mehta S, Wang D, Kuo C-L, Mu J, Vich ML, Allareddy V, Tadinada A, Yadav S (2021) Long-term effects of mini-screw-assisted rapid palatal expansion on airway. Angle Orthod 91:195–205. https://doi.org/10.2319/062520-586.1
Lotfi V, Ghoneima A, Lagravere M, Kula K, Stewart K (2018) Three-dimensional evaluation of airway volume changes in two expansion activation protocols. Int Orthod 16:144–157. https://doi.org/10.1016/j.ortho.2018.01.001
Chang DT, Zhou YH, Liu WT (2017) Evaluation of cone-beam computed tomography on upper airway changes after alternating rapid palatal expansion and constriction. Beijing Da Xue Xue Bao Yi Xue Ban 49:685–690
Miranda F, Garib D, Pugliese F, da Cunha Bastos JC, Janson G, Palomo JM (2022) Upper airway changes in Class III patients using miniscrew-anchored maxillary protraction with hybrid and hyrax expanders: a randomized controlled trial. Clin Oral Investig 26:183–195. https://doi.org/10.1007/s00784-021-03989-3
Alhammadi MS, Elfeky HY, Fayed MS, Ishaq RAR, Halboub E, Al-mashraqi AA (2019) Three-dimensional skeletal and pharyngeal airway changes following therapy with functional appliances in growing skeletal Class II malocclusion patients: A controlled clinical trial. J Orofac Orthop 80:254–265. https://doi.org/10.1007/s00056-019-00185-7
Thereza-Bussolaro C, Oh HS, Lagravère M, Flores-Mir C (2019) Pharyngeal dimensional changes in class II malocclusion treatment when using Forsus® or intermaxillary elastics - An exploratory study. Int Orthod 17:667–677. https://doi.org/10.1016/j.ortho.2019.08.023
Ning R, Guo J, Martin D (2022) Effect of premolar extraction on upper airway volume and hyoid position in hyperdivergent adults with different mandibular length. Am J Orthod Dentofac Orthop 161:E390–E399. https://doi.org/10.1016/j.ajodo.2021.01.027
Abdalla Y, Brown L, Sonnesen L (2019) Effects of rapid maxillary expansion on upper airway volume: A three-dimensional cone-beam computed tomography study. Angle Orthod. https://doi.org/10.2319/101218-738.1
Almuzian M, Ju XG, Almukhtar A, Ayoub A, Al-Muzian L, McDonald JP (2018) Does rapid maxillary expansion affect nasopharyngeal airway? A prospective Cone Beam Computerised Tomography (CBCT) based study. Surg-J R Coll Surg E 16:1–11. https://doi.org/10.1016/j.surge.2015.12.006
Chang Y, Koenig LJ, Pruszynski JE, Bradley TG, Bosio JA, Liu D (2013) Dimensional changes of upper airway after rapid maxillary expansion: A prospective cone-beam computed tomography study. Am J Orthod Dentofac Orthop 143:462–470. https://doi.org/10.1016/j.ajodo.2012.11.019
El H, Palomo JM (2014) Three-dimensional evaluation of upper airway following rapid maxillary expansion: A CBCT study. Angle Orthod 84:265–273. https://doi.org/10.2319/012313-71.1
Iwasaki T, Saitoh I, Takemoto Y, Inada E, Kakuno E, Kanomi R, Hayasaki H, Yamasaki Y (2013) Tongue posture improvement and pharyngeal airway enlargement as secondary effects of rapid maxillary expansion: A cone-beam computed tomography study. Am J Orthod Dentofac Orthop 143:235–245. https://doi.org/10.1016/j.ajodo.2012.09.014
Kim SY, Park YC, Lee KJ, Lintermann A, Han SS, Yu HS, Choi YJ (2018) Assessment of changes in the nasal airway after nonsurgical miniscrew-assisted rapid maxillary expansion in young adults. Angle Orthod 88:435–441. https://doi.org/10.2319/092917-656.1
Zeng J, Gao X (2013) A prospective CBCT study of upper airway changes after rapid maxillary expansion. Int J Pediatr Otorhinolaryngol 77:1805–1810. https://doi.org/10.1016/j.ijporl.2013.07.028
Yilmaz BS, Kucukkeles N (2015) Skeletal, soft tissue, and airway changes following the alternate maxillary expansions and constrictions protocol. Angle Orthod 85:117–126. https://doi.org/10.2319/092713-705.1
Yi F, Liu S, Lei L, Liu O, Zhang L, Peng Q, Lu Y (2020) Changes of the upper airway and bone in microimplant-assisted rapid palatal expansion: A cone-beam computed tomography (CBCT) study. XST 28:271–283. https://doi.org/10.3233/XST-190597
Gianoni-Capenakas S, Flores-Mir C, Vich ML, Pacheco-Pereira C (2021) Oropharyngeal 3-dimensional changes after maxillary expansion with 2 different orthodontic approaches. Am J Orthod Dentofac Orthop 159:352–359. https://doi.org/10.1016/j.ajodo.2020.05.013
Fastuca R, Perinetti G, Zecca PA, Nucera R, Caprioglio A (2015) Airway compartments volume and oxygen saturation changes after rapid maxillary expansion: A longitudinal correlation study. Angle Orthod 85:955–961. https://doi.org/10.2319/072014-504.1
Caprioglio A, Meneghel M, Fastuca R, Zecca PA, Nucera R, Nosetti L (2014) Rapid maxillary expansion in growing patients: correspondence between 3-dimensional airway changes and polysomnography. Int J Pediatr Otorhinolaryngol 78:23–27. https://doi.org/10.1016/j.ijporl.2013.10.011
Niu X, Motro M, Will LA, Cornelis MA, Cattaneo PM (2021) Does rapid maxillary expansion enlarge the nasal cavity and pharyngeal airway? A three-dimensional assessment based on validated analyses. Orthod Craniofac Res 24(Suppl 2):124–133. https://doi.org/10.1111/ocr.12526
Aljawad H, Lee K-M, Lim H-J (2021) Three-dimensional evaluation of upper airway changes following rapid maxillary expansion: A retrospective comparison with propensity score matched controls. PLoS One 16:e0261579. https://doi.org/10.1371/journal.pone.0261579
Ribeiro ANC, de Paiva JB, Rino-Neto J, Illipronti-Filho E, Trivino T, Fantini SM (2012) Upper airway expansion after rapid maxillary expansion evaluated with cone beam computed tomography. Angle Orthod 82:458–463. https://doi.org/10.2319/030411-157.1
DiCosimo C, Alsulaiman AA, Shah C, Motro M, Will LA, Parsi GK (2021) Analysis of nasal airway symmetry and upper airway changes after rapid maxillary expansion. Am J Orthod Dentofac Orthop 160:695–704. https://doi.org/10.1016/j.ajodo.2020.06.038
Chen X, Liu D, Liu J, Wu Z, Xie Y, Li L, Liu H, Guo T, Chen C, Zhang S (2015) Three-Dimensional Evaluation of the Upper Airway Morphological Changes in Growing Patients with Skeletal Class III Malocclusion Treated by Protraction Headgear and Rapid Palatal Expansion: A Comparative Research. PLoS One 10:e0135273. https://doi.org/10.1371/journal.pone.0135273
Pamporakis P, Nevzatoglu S, Kucukkeles N (2014) Three-dimensional alterations in pharyngeal airway and maxillary sinus volumes in Class III maxillary deficiency subjects undergoing orthopedic facemask treatment. Angle Orthod 84:701–707. https://doi.org/10.2319/060513-430.1
Liu Y, Yang K (2021) Three-dimensional changes in the upper airway and craniomaxillofacial morphology of patients with Angle Class III malocclusion treated with a Frankel III appliance. BMC Oral Health 21:634. https://doi.org/10.1186/s12903-021-02013-0
Nguyen T, De Clerck H, Wilson M, Golden B (2015) Effect of Class III bone anchor treatment on airway. Angle Orthod 85:591–596. https://doi.org/10.2319/041614-282.1
Iwasaki T, Takemoto Y, Inada E, Sato H, Saitoh I, Kakuno E, Kanomi R, Yamasaki Y (2014) Three-dimensional cone-beam computed tomography analysis of enlargement of the pharyngeal airway by the Herbst appliance. Am J Orthod Dentofac Orthop 146:776–785. https://doi.org/10.1016/j.ajodo.2014.08.017
Li L, Liu H, Cheng HJ, Han YZ, Wang CL, Chen Y, Song JL, Liu DX (2014) CBCT Evaluation of the Upper Airway Morphological Changes in Growing Patients of Class II Division 1 Malocclusion with Mandibular Retrusion Using Twin Block Appliance: A Comparative Research. PLoS One 9. ARTN e94378. https://doi.org/10.1371/journal.pone.0094378
Temani P, Jain P, Rathee P, Temani R (2016) Volumetric changes in pharyngeal airway in Class II division 1 patients treated with Forsus-fixed functional appliance: A three-dimensional cone-beam computed tomography study. Contemp Clin Dent 7:31. https://doi.org/10.4103/0976-237X.177100
Erbas B, Kocadereli I (2014) Upper airway changes after Xbow appliance therapy evaluated with cone beam computed tomography. Angle Orthod 84:693–700. https://doi.org/10.2319/072213-533.1
Oliveira PM, Cheib-Vilefort PL, de Pársia GH, Melgaço CA, Franchi L, McNamara JA, Souki BQ (2020) Three-dimensional changes of the upper airway in patients with Class II malocclusion treated with the Herbst appliance: A cone-beam computed tomography study. Am J Orthod Dentofac Orthop 157:205–211. https://doi.org/10.1016/j.ajodo.2019.03.021
Abdalla Y, Brown L, Sonnesen L (2020) Effects of a fixed functional appliance on upper airway volume: A 3-dimensional cone-beam computed tomography study. Am J Orthod Dentofac Orthop 158:40–49. https://doi.org/10.1016/j.ajodo.2019.07.013
Chou AHK, Park JH, Shoaib AM, Lee N-K, Lim HJ, Abdulwhab AA, Alfawaz F, Kook Y-A (2021) Total maxillary arch distalization with modified C-palatal plates in adolescents: A long-term study using cone-beam computed tomography. Am J Orthod Dentofac Orthop 159:470–479. https://doi.org/10.1016/j.ajodo.2020.02.011
Xiao S-L, Wu Y-N, Ma R, Li J-H (2020) Effect of invisalign on anterior and posterior upper airway and maxillary bone changes in the treatment of high-angle skeletal Class II malocclusion. Shanghai Kou Qiang Yi Xue 29:410–413
Abdalla Y, Kiliaridis S, Sonnesen L (2022) Airway changes after fixed functional appliance treatment in children with and without morphologic deviations of the upper spine: A 3-dimensional CBCT study. Am J Orthod Dentofacial Orthop S0889–5406(21):00835. https://doi.org/10.1016/j.ajodo.2021.01.029
Park JH, Kim S, Lee Y-J, Bayome M, Kook Y-A, Hong M, Kim Y (2018) Three-dimensional evaluation of maxillary dentoalveolar changes and airway space after distalization in adults. Angle Orthod 88:187–194. https://doi.org/10.2319/121116-889.1
Pliska BT, Tam IT, Lowe AA, Madson AM, Almeida FR (2016) Effect of orthodontic treatment on the upper airway volume in adults. Am J Orthod Dentofac Orthop 150:937–944. https://doi.org/10.1016/j.ajodo.2016.05.013
Valiathan M, El H, Hans MG, Palomo MJ (2010) Effects of extraction versus non-extraction treatment on oropharyngeal airway volume. Angle Orthod 80:1068–1074. https://doi.org/10.2319/010810-19.1
Stefanovic N, El H, Chenin DL, Glisic B, Palomo JM (2013) Three-dimensional pharyngeal airway changes in orthodontic patients treated with and without extractions. Orthod Craniofac Res 16:87–96. https://doi.org/10.1111/ocr.12009
Joy A, Park J, Chambers DW, Oh H (2020) Airway and Cephalometric Changes in Adult Orthodontic Patients After Premolar Extractions. Angle Orthod 90:39–46. https://doi.org/10.2319/021019-92.1
Chen W, Liu Y-H, Xu Q (2018) Effect of maximum anchorage extraction on upper airway in adolescent patients with bimaxillary protrusion. Shanghai Kou Qiang Yi Xue 27:419–423
Guo R, Wang S, Zhang L, Li L, Yu Q, Huang Y, Li W (2022) Oropharynx and hyoid bone changes in female extraction patients with distinct sagittal and vertical skeletal patterns: a retrospective study. Head Face Med 18:31. https://doi.org/10.1186/s13005-022-00334-1
Zhang J, Chen G, Li W, Xu T, Gao X (2015) Upper Airway Changes after Orthodontic Extraction Treatment in Adults: A Preliminary Study using Cone Beam Computed Tomography. PLoS ONE 10:e0143233. https://doi.org/10.1371/journal.pone.0143233
Shi X, Chen H, Lobbezoo F, Berkhout E, de Lange J, Guo J, Aarab G (2021) Effects of miniscrew-assisted orthodontic treatment with premolar extractions on upper airway dimensions in adult patients with Class II high-angle malocclusion. Am J Orthod Dentofac Orthop 159:724–732. https://doi.org/10.1016/j.ajodo.2020.02.016
Author information
Authors and Affiliations
Contributions
Authors to be included and their respective contributions to the review:
Steegman RM
r.m.steegman@umcg.nl
0000-0002-0741-7971
Designing the review; data collection; data management; analysis of data; interpretation data; writing the protocol or review
Renkema AM
a.renkema@umcg.nl
0000-0002-8832-4899
Interpretation data; writing the protocol or review
Schoeman A
a.schoeman@umcg.nl
data collection; data management; analysis of data
Kuijpers-Jagtman, AM
a.m.kuijpers-jagtman@umcg.nl
0000-0001-8172-0090
Coordinating the review; analysis of data; interpretation data; writing the protocol or review
Ren, Y
y.ren@umcg.nl
0000-0002-2374–1771
Conceiving the review; designing the review; coordinating the review; data management; analysis of data; interpretation data; writing the protocol or review
Corresponding author
Ethics declarations
Ethical approval
Not Applicable.
Informed consent
Not Applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Five bar graphs of the relative changes in the airway after different orthodontic interventions.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Steegman, R.M., Renkema, AM., Schoeman, A. et al. Volumetric changes in the upper airway on CBCT after dentofacial orthopedic interventions - a systematic review. Clin Oral Invest 27, 5737–5754 (2023). https://doi.org/10.1007/s00784-023-05207-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-023-05207-8