Abstract
Objectives
To comparatively evaluate the in vivo outcome of MTA repair for contaminated and non-contaminated furcation perforations (FP) with or without PRF and CGF as a matrix in dogs’ teeth.
Methods
Ninety dog teeth were divided into five groups based on the iatrogenic FP repair approach after doing root canal treatment: negative control (without FP), positive control (FP without repair), MTA, MTA + PRF and MTA + CGF groups, where FP were repaired promptly in subdivision 1 (n = 10; non-contaminated) and after 4 weeks of oral contamination in subdivision 2 (n = 10;contaminated). After 3 months, the perforation site was assessed radiographically (vertical bone density), histologically (inflammatory cell count, epithelial proliferation, cementum and bone deposition) and immunohistochemically (OPN and TRAP antibodies localisation). Data collected were statistically analysed using SPSS software at a 0.05 significance level.
Results
The MTA + PRF and MTA + CGF groups demonstrated significantly more bone formation, OPN immunolocalisation and fewer inflammatory cell counts than MTA group. MTA, MTA + PRF and MTA + CGF groups showed significantly favourable radiographic, histological and immunohistochemical healing features than the positive control, especially in non-contaminated subdivisions, that significantly showed better features than the contaminated subdivisions (P < 0.001).
Conclusion
The use CGF and PRF as a matrix beneath MTA in FP repair in dog’s teeth is promising as it could increase hard and soft tissue regeneration in non-contaminated and contaminated perforations.
Clinical relevance
The repair of FP is challenging especially when associated with contaminated inter-radicular bone loss. Radiographic, histological and immunohistochemical comprehensive evaluation of the root and surrounding attachment apparatus response to different perforation repair protocols could give a predictable clinical outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Different procedural errors could occur during root canal treatment (RCT) phases. These errors are considered critical when they hinder the three-dimensional disinfection and seal of the root canal space, thus, causing RCT failure [1]. An artificial communication between the internal root canal system and the external surface of the root is what defines a root perforation [1, 2]. Root furcation perforation is a common endodontic mishap in multirooted teeth, which could induce an immune-inflammatory response, leading to the destruction of the surrounding periodontal ligament, bone resorption and eventually tooth loss [3]. Accordingly, understanding the biological outcome of perforation repair is of prime importance [4]. The best treatment outcome is a perforation seal with reparative mineralised tissue development and the restoration of periodontal attachment. Several host and treatment variables influence the tissue regeneration and long-term success of a perforation repair including the duration of septic exposure, the perforation location and the repair material physiochemical properties [5,6,7,8,9].
Mineral trioxide aggregate (MTA) has been shown in multiple trials to be superior to most endodontic materials for optimal root perforation healing because of its propensity to induce hard and periodontal tissues regeneration [5, 10, 11]. MTA has also showed low bacterial leakage, good biocompatibility and adaptation to cavity walls. Accordingly, it has become the gold standard regenerative material of choice in sealing the root sub-crestal furcal perforations [5, 12].
However, the tooth discolouration, high cost and long setting time (3 h) are some known disadvantages of MTA. Also, MTA had shown cohesive fracture pattern and low values of shear bond strength with immediate definitive restorations [13,14,15,16]. Besides, a major difficulty during non-surgical perforation repair is the poor handling character of MTA. This may result in the repair material being extruded into the periodontal space, interfering with peri-radicular tissue recovery and attachment [17, 18].
Therefore, it was suggested to place a barrier (matrix) in the perforation site against which the MTA repair material could be compacted [12, 18]. Calcium sulphate and absorbable collagen-based material such as Collaplug were used previously as matrices. However, both materials have no inducting effect on periodontal and bone tissue regeneration. Besides, calcium sulphate was shown to affect the sealing ability of MTA and caused unfavourable inflammatory reaction [8, 18]. Collaplug, on the other hand, did not affect the sealing ability of MTA but was not a good barrier to prevent overextension since it was not pressure resistant [18].
In endodontic regeneration techniques, platelet concentrates have been advocated as a scaffold (matrix) [19,20,21,22]. Choukroun et al. [23] described platelet-rich fibrin (PRF) in 2006 as a second-generation platelet concentrate to address the drawbacks of first-generation platelet-rich plasma (PRP). The PRF preparation could be done with minimal experience because it is a simple single step not, as PRP, that requires a two-step centrifugation, purification and coagulant addition [24].
Because of the delayed fibrin polymerisation during PRF processing, platelet cytokines, glycanic chains and growth factors are intrinsically incorporated into the fibrin meshes [23, 24]. Through its inherent growth factors, PRF has been proven to improve the regeneration of dental soft and hard tissues [23, 25].
Concentrated growth factors (CGF), a third-generation platelet concentrate, were reported to have an advantage over PRP and PRF in terms of cell proliferation and osteoblastic differentiation, as well as a higher growth factor concentration [26, 27]. CGF induced more cell migration, chemotactic activity on inflammatory cells, angiogenesis and tissue remodelling than PRF due to its unique centrifugation technique [27, 28]. Moreover, CGF successfully promoted the osseous healing of extensive periapical bone lesion and increased the regenerative rate in surgical endodontic treatment methods [28, 29]. Because of these outstanding regenerative abilities of PRF and CGF, they were selected in the current study as an alternative internal matrix material to calcium sulphate and collagen-based matrices during MTA furcation perforation repair.
Thorough review of the literature revealed a scarcity of papers on the in vivo use of CGF in perforation repair [5, 28, 30]. The comparative effect of using either CGF or PRF on MTA perforation repair is still unclear. Also, immune histochemical evaluation of the MTA perforation repair with or without PRF or CGF as internal matrices in contaminated and non-contaminated furcation perforations was not previously done. As a result, the current in vivo study aimed to compare the healing outcomes (radiographic, histologic and immunohistochemical assessment) of MTA repair for contaminated and non-contaminated furcation perforations in dogs’ teeth with or without PRF and CGF as a matrix. The null hypothesis in this study was that there were no significant differences in the healing response to MTA with or without PRF or CGF in the repair of contaminated and non-contaminated experimental perforation defects in dog’s teeth.
Methods
Ethical approval
The Institutional Research Ethics Committee (REC), Faculty of Dentistry, Suez Canal University, Egypt, reviewed and approved this work (No.335/2021). All methods are reported in accordance with ARRIVE guidelines for the reporting of animal experiments (https://www.arriveguidelines.org).
Animal model selection
Dogs were chosen for this study from the animal house at Suez Canal University’s Faculty of Veterinary Medicine. These dogs were used in this scientific purpose because they had already been requested and slated for euthanasia for veterinary reasons (e.g. behavioural issues, life-changing circumstances, convenience, overpopulation). Every attempt was made to reduce animal suffering and the quantity of used animals.
Sample size calculation:
A repeated measures analysis of variance (repeated measures ANOVA) was proposed to analyse and compare the effect of MTA, MTA + PRF and MTA + CGF on the healing of contaminated and non-contaminated perforation defects in dog teeth.
G*Power version 3.1.9.2 (University Kiel, Germany. 1992–2014) was used to calculate the sample size [31]. The effect size d was 0.38 when the alpha (α) level was 0.05, and the beta (β) level was 0.05, implying that power = 80%; the estimated sample size (n) should be at least 90 samples. Each treatment modality (group II, III, IV, V) was represented by 20 samples, and the negative control (group I) was represented by only ten samples. Each group (excluding the negative control group) was further separated into two equal subgroups (with ten samples each, n = 10), representing the perforation status (contaminated or non-contaminated).
Sample selection and grouping:
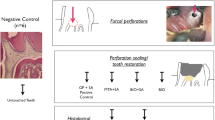
Three healthy premolar teeth were chosen from each quadrant of eight healthy mature male mongrel dogs, ageing 14–16 months (12 teeth/dog × 8 = 96 teeth ~ 90). The selected teeth should have mature roots, intact crowns and without any periodontal defects. Preoperative assessment was also done using the preapical radiographic method to exclude any tooth with root and/or bony defect at the furcation area. Teeth were randomly separated into five groups (two control and 3 experimental groups) using a web program (http://www.random.org/): negative control (I), positive control (II), MTA (III), MTA + PRF (IV) and MTA + CGF (V) groups. Each experimental group, except the negative control group I (n = 10), was further separated into two subdivisions (n = 10) based on perforation status: (1) contaminated perforations and (2) non-contaminated perforations (Fig. S1).
Dog anaesthesia:
Food was withheld for 6–8 h prior to treatment. Each dog was premedicated 15 min before general anaesthesia induction with an intramuscular injection of 1 mg/kg chlorpromazine hydrochloride (Hikma Pharmaceuticals, London, UK). General anaesthesia was achieved and maintained with intravenous administration of a 2.5% solution of thiopental sodium (EPICO, Cairo, Egypt) until the major reflexes were abolished [32].
Teeth handling
Root canal treatment
The oral cavity was disinfected with povidone-iodine (Betadine, 10%, Mundi Pharma, Cairo, Egypt). Rubber dam (TOR VM, Moscow, Russia) was used to isolate the teeth by applying the rubber dam clamp (butterfly #210 or # 202) + sheet first, followed by the rubber dam frame (Fig. S2). Additional seal with light-cured resin barrier (Opal Dam, Ultra Dent, South Jordan, USA) around the tooth neck (CEJ) was done. The crown and rubber dam sheet surface were disinfected with 30% hydrogen peroxide (H2O2, LUNA pharmaceutical, Cairo, Egypt), followed by 2.5% sodium hypochlorite (NaOCl, DEXA company for chemicals, Cairo, Egypt).
In all included teeth, a standard endodontic access cavity was performed with a sterile round, Endo-Z burs (Dentsply Maillfer, Ballaigues, Switzerland), and water cooling. Patency was tested with a #10 k-file (Micro Mega, Besancon, France). The working length was 1 mm from the apex using a Root ZX electronic apex locator (J Morita Corp, Tokyo, Japan). The canal orifice was opened and flared in an anti-curvature motion with Fanta blue rotary orifice opener files (#17/12, Fanta, TX, USA). Root canal instrumentation was done using Fanta blue files up to AF4 35/04. Irrigation of each root canal was done with 2 mL sodium hypochlorite 2.5% between each file use. A final rinse of 3 mL of saline 9% (Otsuka pharmaceutical, Shanghai, China) and 3 mL of EDTA 17% solution (Prevest Denpro Limited Company, Jammu, India) was performed after canal instrumentation. The canals were subsequently dried using paper points #35/04 (Dentsply, Tulsa, USA) and obturated using a single cone technique with gutta-percha (#35/04) (Dentsply, Tulsa, USA) and AH plus sealer (Dentsply, Konstanz, Germany).
Experimental furcation perforation creation
In the teeth of contaminated perforation (subdivision 1) (n = 40) from the three experimental (MTA, MTA + PRF, MTA + CGF) and positive control groups, contaminated furcation perforations were created as follows: Each perforation was done at the floor of the pulp chamber (midway between mesial and distal canal orifices) using a high-speed long shank round bur # 4 (#4RC; SybronEndo Europe, Amersfoort, Netherlands) with coolant. The perforation width was standardised to 1.5 mm, and its length was extended to 2 mm in the alveolar bone [8]. Radiograph was taken to check the correct position and depth of furcation perforation. The access cavities and the created perforation sites were left without any coronal seal for 4 weeks to allow bacterial contamination from saliva in the mouth cavity to cause infection and inflammation. For pain and infection control, the dogs were given 10 mg/kg intramuscular cefotaxime sodium (Wockhardt UK Ltd, Wrexham, UK) and 1.1 mg/kg diclofenac sodium (Voltaren, Novartis Pharma, Basel, Switzerland) once a day for 5 days following surgery.
On the other hand, in the teeth of non-contaminated perforation (subdivision 2) (n = 40) from the three experimental (MTA, MTA + PRF, MTA + CGF) and positive control groups, after doing the root canal treatment, the access cavity was sealed with sterile cotton and a temporary filling (Coltosol F: Coltosol Whaledent, Altstatten, Switzerland) without developing any furcation perforation.
After 4 weeks, in contaminated perforations (subdivision 1), periapical radiographs were taken to confirm the presence of bone resorption and a lesion at the furcation area. While in non-contaminated perforations (subdivision 2), the temporary filling was removed, and an immediate furcation perforation was performed in the same manner as indicated in subdivision 1 but without oral contamination.
Treatment modalities for perforation repair
The access cavities and the perforations were flushed with 2.5% sodium hypochlorite, followed by 9% normal saline. Excavator was used to curet the perforation site to remove any inflammatory tissues or debris. The bleeding was stopped by washing the region with 9% sterile saline solution and applying pressure with wet cotton pellets [8, 17, 33, 34]. In the two subdivisions of the positive control group (group II), the experimental perforation was left without any perforation repair material.
MTA repair (G.III)
In the MTA group (III), the perforation was repaired with MTA (white MTA Angelus, Londrina, PR, Brazil) non-surgically through the perforation as follows: The MTA mix was made according to the manufacturer’s instructions. Using the microapical placement device (MAP system, Produits Dentaires SA, Vevey, Switzerland), the mix was carefully distributed in increments to seal the perforation location (the pulp chamber floor). Hand pluggers (Buchanan pluggers; SybronEndo Europe, Amersfoort, Netherlands) were used to generate a 3-mm-thick MTA plug after light condensation of the MTA. For 10 min, a moist cotton pellet soaked in distilled water was placed over the repair MTA plug to allow for setting via hydration reaction [35].
PRF + MTA (G.IV) and CGF + MTA repair (G. V)
In groups (IV) and (V), PRF and CGF were prepared to be placed as matrices in the perforation defects non-surgically before MTA placement as follows (Fig. S3):
Preparation of PRF [24, 27, 36]
Ten millilitres of autologous venous blood was collected from the cubital region of the dog’s forearm in a 10-mL sterile test tube without anticoagulant and immediately spun at 3000 rpm for 12 min in a table-top centrifuge. The final product had three layers: (a) RBC at the bottom, (b) PRF clot in the middle and (c) platelet-deficient plasma in the uppermost layers (PPP). The central clot’s platelet-rich fibrin (PRF) was carefully extracted with a tweezer.
Preparation of CGF [26,27,28,29, 36,37,38]
As described in the PRF, but the autologous venous blood was centrifuged using a one-step centrifugation methodology (Medifuge, Silfradent, Sofia, Italy): 30-s acceleration, 2 min 2700 rpm, 4 min 2400 rpm, 4 min 2700 rpm, 3 min 3000 rpm, 36-s deceleration and stop. Below the dividing line, the ensuing intermediate layer comprising CGF was taken.
Treatment of the experimental groups (MTA + PRF and MTA + CGF) with the matrices
Pieces of PRF and CGF with average standardised size of 1.5 × 2 mm were packed, respectively, in groups MTA + PRF (IV) and MTA + CGF (V) into their corresponding interradicular area (bone and periodontal defects) by using hand pluggers to the level of cementoenamel junction (CEJ). After that, a 3-mm MTA plug was packed over the platelet concentrates matrix. The access cavity in all groups was definitively sealed with glass ionomer filling (Medifill: Promedica, Neumunster, Germany). After doing different treatment modalities for perforation repair, periapical radiographs were taken immediately for all teeth using a digital sensor (EzSensor Classic, Vatech, Gyeonggi-do Korea). A Rinn (XCP) sensor holder (Rinn, Dentsply Sirona, Charlotte, USA) was employed to achieve image consistency for the immediate postoperative and follow-up radiographic fields. A small individual occlusal stent was fabricated from acrylic resin (Duralay, Reliance, Alsip, USA) to be used for setting the sensor at a standard angulation in relation to the teeth [39]. Images were captured using an X-ray imaging system (Fischer, Sindelfingen, Germany).
Radiographs for all groups and subgroups were saved as a baseline for later comparison after the follow-up period. Dogs were housed in the animal home at Suez Canal University’s Faculty of Veterinary Medicine for three months, providing follow-up care as described previously [11].
Methods of evaluation
After 3 months, the dogs were euthanised with an intravenous barbiturate overdose of 6% pentobarbital (120 mg/kg, Nembutal, Akorn, Lake Forest, USA) [8, 40].
Radiographic evaluation
Follow-up radiographs were taken for all teeth in the same way described previously. To quantitatively estimate the bone density at the perforation site (vertical bone at the inter radicular area), the radiographic images were analysed using Digora image analysis software (Digora for windows, Soredex, Tuusula, Finland) (Fig. S4). The degree of density ranged from zero, representing black, to 239, representing white. To avoid intra-observer error, the same radiologist performed the study on two distinct occasions separated by 1 week. The results of both trials were combined, and the mean was computed.
The change in vertical bone density in the follow-up radiographs in comparison to the baseline radiographs was expressed as percentages (%) calculated from the following equation:
Histological evaluation
The jaws (maxillae and mandibles) were removed, dissected and perfused with 10% buffered formalin solution (MilliporeSigma, Burlington, MA, USA) for 8 weeks for fixation. After fixation, the specimens were decalcified for 10 weeks in formic acid-sodium citrate (Pioneer Research Chemicals Ltd, Essex, UK) [41]. Each tooth with surrounding bone was separated through lancet (to obtain individual blocks containing the teeth and adjacent peri-radicular tissues) then washed with saline. Specimens were then sectioned at 4–6 µm (μm) in a longitudinal manner parallel to the mesiodistal plane using previously published method [42]. A histopathologist blindly examined serial sections through the perforation that was mounted on glass slides, deparaffinised, hydrated and stained with haematoxylin and eosin. The following criteria were used to evaluate the histology:
-
a.
Inflammatory cell count [43]: Image analysis software (Image J, 1.41a®, NIH, USA) was used to calculate the average inflammatory cell count of three specific microscopic fields. After selecting the range of inflammatory cell size from 457 to 4450 pixels and circularity from 0.3 to 1, the inflammatory cells were counted. All photographs were taken with a digital camera (C5060, Olympus, Tokyo, Japan) linked to a light microscope at a magnification of × 400 (Fig. 1).
-
b.
New cementum deposition was measured as follows [12]: score 0: no new cementum deposition, 1: newly formed cementum deposition on lateral walls of perforation or close to it, 2 and 3: partial and entire newly produced cementum barrier, respectively.
-
c.
New bone formation was assessed as followings [43]: For each section, three microscopic fields at an original magnification of × 20 were acquired. The newly produced bone was detected beneath the perforation site, and the image was transformed into an 8-bit format. Image analysis software (Image J, 1.41a®, NIH, USA) was used to assess the area percentage of new bone after adjusting a colour threshold to the newly produced bone (Fig. 2). The mean of the three fields for each segment was calculated and reported. To remove intra-examiner variability, all examinations were redone after 1 week by the same histopathologist. The results of both exams were combined, and the mean was computed.
-
d.
Epithelial proliferation [11, 44]: the frequency of epithelial proliferation presence or absence in each group was recorded and calculated as a percentage (%) (number of teeth specimens with epithelial proliferation (N) / total specimens (10) × 100).
Representative light microscopic photomicrographs (H&E, × 400) for the inflammatory cell count phases are as follows: (A) the original image. (B) The original image after grayscale conversion. (C) The image threshold and colour coding used to select the majority of the cells in the image. (D) The software generated a thresholded black and white binary image automatically. (E) Inflammatory cells count following removal of unwanted cells (based on size and circularity)
The immunohistochemical (IHC) detection system
In this study, the expression of osteopontin (OPN) and tartrate-resistant acid phosphatase (TRAP) antibodies in the perforation site was demonstrated immunohistochemically [45]. IHC staining was performed by a standard avidin-biotinperoxidase procedure by using the Ultravision Polyvalent (Rabbit-Mouse) Horseradish Peroxidase (HRP) Kit, 125 mL (Thermo Fisher Scientific, Waltham, MA, USA). The sections were incubated with pre-diluted OPN polyclonal antibody (Master Diagnóstica, Granada, Spain) to identify cellular and interstitial expression. Sections were also incubated with rabbit anti-TRAP/TRAP polyclonal antibody (TRAP, Abcam, Cambridge, MA, USA) overnight at 4 °C.
After rinsing the sections thoroughly with PBS, the sections were incubated with a biotinylated secondary antibody (Master Diagnóstica, Granada, Spain). Then the horseradish peroxidase-conjugated streptavidin solution (Thermo Fisher Scientific, Waltham, MA, USA) was added and incubated at room temperature for 10–15 min. Finally, the sections were visualised with diaminobenzidine substrate (DAB) (diaminobenzidine chromogen and substrate system) 125 mL (Thermo Fisher Scientific, Waltham, MA, USA) as chromogen for 3–5 min at room temperature. The slides were processed immunohistochemically at the same laboratory conditions to obtain comparable staining intensities. The immune-expressions (optical density) of OPN and TRAP together with TRAP-positive cell detection were measured using the J Image analyser computerised system to determine the early bone formation and resorption in the perforation area.
Statistical analysis
The intra-observer agreement for the radiographic, histologic and immunohistochemical observations was calculated using kappa coefficient. Level of agreement was suggested according to the kappa results as follows: values ≤ 0 as indicating no agreement and 0.01–0.20 as none to slight, 0.21–0.40 as fair, 0.41–0.60 as moderate, 0.61–0.80 as substantial and 0.81–1.00 as almost-perfect agreement [46, 47].
Analysing data for normalcy entailed evaluating data distribution and using normality tests (Kolmogorov–Smirnov and Shapiro–Wilk tests). Because the data for radiographic bone density, inflammatory cell count, new bone formation, OPN and TRAP expression were parametric, a repeated ANOVA test was used to assess the impact of repair type. When the ANOVA test was significant, the Bonferroni post hoc test was used to compare groups. An independent T-test was utilised to compare the two subgroups within each group.
Because the scores (new cementum deposition) were non-parametric data, they were analysed using non-parametric tests and reported as median and range values. The Kruskal–Wallis test was used to compare groups. Duncan’s test was used for pair-wise comparisons between groups. The Mann–Whitney test was employed to distinguish between the two categories (subgroups). The chi-square test was performed to analyse and compare the frequency of epithelial proliferation in all groups and subgroups. The significance level was set at P ≤ 0.05. Version 23.0 of IBM SPSS Statistics for Windows, IBM Corp., Armonk, NY, was used for statistical analysis.
Results
The anaesthetic or the experimental methods did not harm the dogs. The average kappa values for the investigators’ self-agreement were almost perfect (0.83–0.97). The teeth in group I (negative control) revealed no signs of gingivitis and no changes in inter-radicular bone density.
Radiographic finding (Table 1, Fig. 3)
Representative photomicrographs of radiographic views for the following: (A) negative control group. (B, C) Immediate postoperative and follow-up radiographs for positive control group respectively. (D, E) Immediate postoperative and follow-up radiographs for MTA group respectively. (F, G) Immediate postoperative and follow-up radiographs MTA + PRF group respectively. (H, I) Immediate postoperative and follow-up radiographs for MTA + CGF group respectively in both subdivisions (contaminated and non-contaminated furcation perforations)
Regarding the intergroup comparison, the positive control group (II) showed a significant loss in the mean inter-radicular bone density compared to all study groups. On the other hand, different treatment modalities in groups MTA (III), MTA + PRF (IV) and MTA + CGF (V) demonstrated a statistically significant increase in mean inter-radicular bone density over the positive control group (II) (P < 0.001). Groups MTA + PRF and MTA + CGF showed a non-statistically significant increase in mean bone density compared to the MTA group, independent of subdivision. Still, the follow-up radiographs of these groups (MTA, MTA + PRF and MTA + CGF) showed significantly decreased mean inter-radicular bone density than the negative control group (I). In terms of intragroup comparisons, all teeth of contaminated perforations subdivision (1) showed a less statistically significant increase in mean bone density than those of non-contaminated subdivision (2) in groups MTA, MTA + PRF and MTA + CGF (P = 0.002, < 0.001, 0.012 respectively). However, the positive control group showed no statistically significant variations across subdivisions (P = 0.522).
Histopathological findings (haematoxylin and eosin staining)
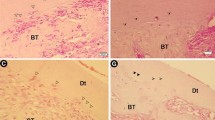
Histologic analysis for the teeth in the negative control group (I) indicated normal alveolar bone with osseous remodelling. Trabecular spaces were typically occupied by fatty bone marrow and blood arteries/arterioles. There were no inflammatory alterations in the periodontium, and the periodontal ligament fibres were oriented obliquely (Fig. 4A).
Representative photomicrographs showing (A) negative control with normal histological picture of inter-radicular bone “B”, intradicular fibres “IR”, dentine “D”, cementum “C”. (B) Contaminated positive control epithelial proliferation “black arrow”, site of perforation “Pr”, inflammatory cell infiltration, dissociation of collagen fibres and vacuolisation of the connective tissue of inter-radicular area. (C) Non-contaminated positive control, high grad inflammatory cell infiltration, collagen fibre dissociation, resorption of inter-radicular bone with widening of bone marrow cavities “blue arrows”. Notice: The resorption of the cementum of the root “black arrows”. (D) Contaminated MTA, high-grad inflammatory cell infiltrations, collagen bundle interlacing with the inflammatory cells “red arrow”. Epithelial cell proliferation “blue arrows”. Resorption of inter-radicular bone. (E) Non-contaminated MTA with inflammatory cell infiltration. Osteoid matrix with osteoblastic rimming “black arrows”. Focal areas of cementum deposition at furcation site “blue arrows”. (F) Contaminated MTA + PRF group with inflammatory cell infiltration, bone trabeculae interlace with collagen fibres” (black arrows”. (G) Non-contaminated MTA + PRF group with low-grade inflammatory cell infiltration with cementum deposition mostly sealed the furcal perforation “blue arrows”. Bone trabeculae interlace with the collagen fibres. Notice: formation of new fibres with more organisation and orientation of collagen bundles. (H) Contaminated MTA + CGF group with epithelial proliferation “blue arrow”, inflammatory cell infiltration, bone trabeculae interlace with the collagen fibres “black arrow”. (I) Non-contaminated MTA + CGF group with low-grade inflammatory cell infiltration. Cementum deposition which mostly cover the perforation site “blue arrows”. New fibres with almost organising manner. Bone trabeculae interlace with the collagen fibres. (Mag. × 200–400)
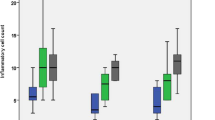
Inflammatory cell infiltration count (Table 2, Fig. 4)
The mean inflammatory cell count in groups MTA (III), MTA + PRF (IV) and MTA + CGF (V) were statistically lower than in the positive control group (II). Still, these groups had significantly higher mean inflammatory cell counts than the negative control group (I). The mean inflammatory cell count in MTA group (III) was statistically higher than in groups MTA + PRF and MTA + CGF. Regarding intragroup comparisons, all non-contaminated subgroups had significantly lower mean inflammatory cell counts than their contaminated counterparts.
New cementum deposition (Table 3, Fig. 4)
The negative and positive control groups showed no new cementum deposition. In both subgroups of groups MTA (III), MTA + PRF (IV) and MTA + CGF (V), the scores of new cementum deposition were significantly greater than in the negative and positive control groups (I, II). At the same time, there were no statistically significant differences between groups MTA, MTA + PRF and MTA + CGF. Despite of the focal cementum depositions that were noticed in some samples of the contaminated subgroups, they were all significantly lower than in the non-contaminated counterparts.
New bone formation (Table 4, Fig. 4)
In groups MTA (III), MTA + PRF (IV) and MTA + CGF (V), there were statistically significant greater mean bone formation values than positive control group (II). Still, all experimental groups (II, III, IV and V) showed significantly lower mean bone formation values than the negative control group (I). In the contaminated subdivisions, MTA + PRF group and MTA + CGF group showed more significant bone formation than MTA group. While in the non-contaminated subdivisions, no statistically significant differences were found between them. All contaminated subdivisions showed significantly fewer new bone formation values than the non-contaminated subgroups.
Epithelial proliferation (Table 5, Fig. 4)
All experimental groups demonstrated a significant increase in the frequency of epithelial proliferation compared to the negative control group (I), while there was significantly less frequent epithelial proliferation in groups MTA, MTA + PRF and MTA + CGF than in the positive control group. At the same time, no significant differences were detected between groups MTA, MTA + PRF and MTA + CGF. The non-contaminated subdivisions showed non-statistically significant less frequency of epithelial proliferation than the contaminated subgroup in all groups.
Immunohistochemical evaluation
Immunohistochemical expression of OPN (Table 6, Fig. 5)
Representative photomicrographs of (A) negative control group showing moderate immunoreactivity to OPN for alveolar bone and periodontal ligaments. (B, C, D) Both positive control subgroups and contaminated MTA groups respectively, showing Faint immunoreactivity to OPN. Notice the OPN expression at border of alveolar bone (black arrow) and border of root cementum (red arrow). (E, F, H) Non-contaminated MTA group, showing moderate immunoreactivity to OPN for PDL and alveolar bone. (G, I) Non-contaminated subgroups of MTA + PRF& MTA + CGF respectively showing intense immunoreactivity to OPN for PDL and alveolar bone. Note: the new bone trabeculae (arrow). (Mag. × 200–400)
OPN values in the periodontal ligaments and alveolar bone of both positive control subgroups showed statistically significantly lower values than the negative control group. OPN values in both subgroups of MTA, MTA + PRF and MTA + CGF groups were statistically significantly higher than the positive control subgroups. Moreover, in non-contaminated subgroups, MTA showed significantly less values than both MTA + PRF and MTA + CGF. There was no difference between MTA + PRF and MTA + CGF subgroups, but they both showed greater significant mean OPN values than negative control.
Immunohistochemical expression of TRAP (Table 7, Fig. 6)
Representative photomicrographs of (A) negative control showing negative immunoreactivity to TRAP. (B, C) Both positive control subgroups showing moderate immunoreactivity to TRAP. (D, E) Both subgroups of MTA showing faint immunoreactivity to TRAP. (F, H) Faint to negative expression to TRAP. (G, I) Negative expression to TRAP. Notice: TRAP.+ multi-nucleated cells (black arrows) and TRAP expression on the root cementum (red arrow). (TRAP antibody Orig. Mag. × 200–400)
Regarding intragroup comparison, the contaminated subgroups showed statistically significant greater values than the non-contaminated subgroups. Contaminated subgroups of MTA, MTA + PRF and MTA + CGF showed no statistical significance with each other’s but demonstrated significant differences between negative and positive groups. Otherwise, non-contaminated MTA, MTA + PRF and MTA + CGF subgroups showed no statistical significance with each other and with negative control. However, they demonstrated a statistically significant less TRAP values than the positive control group. Note: Some TRAP+ multi-nucleated cells were found in both subgroups of the positive control group and MTA group. In contrast, TRAP+ multi-nucleated cells were hardly detectable in both subgroups of MTA + PRF and MTA + CGF groups.
Discussion
MTA was considered a well-established perforation repair material [5, 48]. Though when the furcation perforation is associated with iatrogenic or pathogenic inter-radicular crestal bone destruction, the prognosis may be worsened [8]. Accordingly, the combination of a natural matrix that could deliver growth factors was suggested to enhance the regeneration of the calcified and periodontal tissues at the perforation site [8, 17, 28, 38, 49].
This study has been one of the first in vivo attempts to evaluate the healing response of contaminated and non-contaminated furcation perforation defects to MTA repair using CGF as matrix. Another strength of the present study was the combination of multiple quantitative and qualitative evaluation tools for different perforation repair protocols (MTA, MTA + PRF and MTA + CGF) including radiographic, histologic and immunohistochemical assessments.
Because of the easy accessibility and visibility of the root furcation area, dogs were employed as an in vivo model in this investigation. Thus, allow easy, reproducible experimental perforation creation in the furcation of their well-developed roots [17]. To get a clinically predictable outcome for the healing after different perforation repair strategies in the current study, radiographic image analysis was done using Digora software as recommended previously [38, 43, 49]. Using grey levels, this software finds the differences in pixels across studied locations and yields numerical data about the radiographic bone mineral density.
The normal variations in the bone density between samples would not affect the results of the current study, as the percentage of change in the radiographic bone density was calculated for each tooth in the follow-up radiographs compared to the baseline radiograph as reference [43]. Though, the radiographic images were two-dimensional and require a specific amount of change in the calcified tissues to be apparent radiographically. Moreover, it could not assess the soft tissue reaction to different repair strategies. Accordingly, the histological and immunohistochemical evaluations were additionally used in the current study to overcome the limitations of radiographic assessment. The histological evaluation demonstrated the qualitative hard and soft tissue reaction to different repair strategies, and by using the ImageJ software analysis, the quantitative analysis could also be performed. Besides, the Immune histochemical investigation of TRAP and OPN antibodies showed the osteoclastic and osteoblastic bone cells reaction to different tested treatments.
Osteopontin (OPN) is a secreted non-collagenous bone matrix protein that supports bone remodelling. It is a member of the tiny integrin-binding ligand N-linked glycoprotein family (SIBLING). Pre-osteoblasts, osteoblasts and osteocytes create the most OPN in bone [50]. OPN is generated also by extraosseous cells, odontoblasts, some bone marrow cells, various cultured fibroblasts and epithelial-derived cell lines in addition to bone cells [51]. It was named after its role as a link between cells and minerals. The postulated dual role of OPN in biomineralisation is to increase osteoclast and osteoblast cell attachment. OPN expression was additionally shown within the periodontal ligaments that further support the theory that cells from the periodontal ligament have the ability to facilitate hard tissue formation and consequently play a role in periodontal regeneration [50, 51]. The previous studies explained that the moderate and focal immunolocalisation of OPN in the samples denoted bone resorption, and its intense expression demonstrated bone formation [51].
On the other hand, TRAP staining is unique to mature osteoclasts and is more suited for studying the reaction of calcified tissue [52]. TRAP-positive cells are naturally present due to the high turnover of the alveolar bone, and accordingly, they were found in all study groups of the present investigation. However, the strong release of TRAP by osteoclasts has been demonstrated to correlate with their resorptive behaviour and serves as a selective marker for osteoclastic activity [53].
The current investigation partially rejected the null hypothesis because different treatment techniques for contaminated or non-contaminated furcation perforations mostly produced statistically different results. In the positive control group, no repair material was placed at the experimental perforation site. Accordingly, when comparing this group with others with different perforation repair modalities, these teeth showed the greatest inflammatory cell count and immunolocalisation of TRAP with no new bone or cementum formation. Radiographically, this group presented the greatest loss in inter-radicular bone density.
Besides, regardless of the high rate of epithelisation seen in this study experimental samples (owing to the furcation’s proximity to the CEJ and lacking the root trunk in dogs’ teeth) [17], the positive control group provoked the highest epithelial proliferation prevalence than other groups, denoting healing failure. These results emphasise the importance of combining adequate disinfection and immediate seal of the perforation defect [6, 8, 12, 54] for the success of furcation perforation repair thus, preventing total tooth loss.
On the other hand, the increased bone density in the follow-up radiographs of different perforation repair strategies (MTA, MTA + PRF, MTA + CGF) reflects their success in managing contaminated and non-contaminated perforations. This was also confirmed by the lower inflammatory cell count and TRAP immunolocalisation compared to positive control. In addition to the higher OPN immunolocalisation, bone and cementum deposition, indicating the continuous improvement in hard tissue healing. MTA causes immune cells to release lymphokines, and bone coupling factors, which are essential for cementum repair and regeneration, as well as the healing of osseous periapical defects [55, 56]. PRF and CGF are made up of a fibrin network that is densely packed with blood platelets, cytokines, growth factors and leukocytes [57]. Furthermore, PRF and CGF act as autogenous gelatinous substances that improve clot stability, modulate inflammation and stimulate stem cell chemotaxis, accelerating tissue healing [20, 26,27,28].
In accordance with previous studies [3, 8, 9, 12], the delay in the healing process in the contaminated perforations may result from the severe inflammatory cell infiltration that was shown histologically because of their exposure to microbial infection from the oral environment [58]. Similarly, as a reaction to the increased infection and inflammation, higher immunohistochemical localisation of TRAP and lower intra-radicular bone density, OPN expression, new bone and cementum deposition were found, denoting more resorptive reaction compared to the non-contaminated perforations [12].
Interestingly, in the contaminated perforations repaired with MTA, PRF + MTA or CGF + MTA, few histological samples showed focal areas of new cementum deposition and bone formation. Besides, there were greater OPN immunolocalisation and radiographic bone density when compared to the unrepaired perforations (positive control). The antibacterial and anti-inflammatory character of these materials, additionally augmented by the growth factors content of PRF and CGF, could possibly explain their osteogenic differentiation ability even in inflammatory conditions [59,60,61]. Platelet concentrates have shown to release osteogenic growth factors like BMP-2 (important member of the TGF-β superfamily), mainly at low pH that is the commonly found in the environment of wound healing sites [60, 61]. Furthermore, Ford et al. ascribed the healing response of delayed furcal perforations to successful disinfection with 2.5% sodium hypochlorite prior to repair [62].
On comparing the tested treatment modalities (groups) in the current study, regardless of the status of the perforation site, the application of PRF + MTA and CGF + MTA significantly decreased the inflammatory cell count than using MTA alone. The autologous origin, antimicrobial host defence of platelets and anti-inflammatory activity of PRF and CGF could explain these findings [22, 63]. Likewise, prior research by Shaheen et al. [64] and Dohan et al. [65] had found that MTA had a much greater inflammatory cell count than MTA + PRP and MTA + PRF.
Moreover, different articles in literature including a narrative review [28], systematic review [66], meta-analysis [67], clinical trial [68] and others [64, 65, 69] found that the PRF and CGF, with their natural fibrin framework, could deliver a large number of growth factors to the target site, thus, encouraging angiogenesis and keeping their mineral deposition activity for a relatively more extended period, and stimulating the complex tissue regeneration effectively [70]. This might explain the continuous layer of cementum bridge formation with the increased OPN immunolocalisation in MTA + PRF and MTA + CGF repaired non-contaminated perforations when compared to those repaired with MTA, where only focal areas of cementum deposition in the defect site were noticed.
Generally, MTA + CGF repair showed a non-statistically significant better radiographic, histologic and immunohistochemical healing and regeneration outcomes of than MTA + PRF in the current study. This might be attributed to the alternated and controlled speed mode of centrifugation of CGF that provides a higher chance to collide with the glass wall resulting in more platelet rupture. Accordingly, a fibrin matrix that is larger, denser, with more growth factors contents, increased tensile strength and viscosity than PRF could results [71, 72].
Nityasri et al. [73] reported that CGF is a fibrin tissue adhesive with haemostatic and sealing properties. This provides the wound stability required to attach a new connective tissue to the root surface. CGF was also shown to support cytokine attachment and promote mesenchymal cell-mediated periodontal tissue regeneration achieved. Additionally, it was found in previous research that CGF represented a source of cells with stem features. At the same time, they were able to deliver osteogenic growth factors that upregulated the expressions of Tafazzin (TAZ) and genes related to osteogenic differentiation (BMP-2, RUNX2, COL1a1 and OCN, Col I and OPN) [74]. As a result of the current findings, the CGF fibrin membrane has the potential to act as a periodontal and bone regeneration biomaterial successfully providing new insights into the therapy of furcation perforation via tissue regeneration.
The major limitation of the current study was the short follow-up period (3 months only) because the long-term follow-up may reveal different outcomes [8, 12]. Also, MTA used in the current study has a reduced setting time from 2 h for ProRoot MTA to 10 min for MTA-Angelus. This may impact its physiochemical and biological properties, preventing MTA-Angelus from better wetting and adapting to hollow walls [75]. A previous study recommended matrix presence with MTA-Angelus to improve adaptation.
The MTA’s discoloration potential was another disadvantage, and it was found that MTA composition and blood contact adversely affect the colour stability. Thus, when repairing perforations especially at the CEJ level with platelet concentrates, it is recommended to eliminate or minimise MTA discoloration through ensuring complete haemostasis, blood clot stabilisation and cleaning the blood remnants present at the perforation site as possible before MTA placement [13, 76]. Unfortunately, the different perforation repair protocols’ effect on tooth discoloration was not assessed in the current study. Accordingly, longer follow-up periods using other recently introduced types of calcium silicate cements (that do not include bismuth oxide) are recommended in further studies.
Moreover, it is recommended to use more mineralised and periodontal tissue markers (like alkaline phosphatase (ALP), bone morphogenetic protein (BMP-2), cementum attachment protein (CAP), bone sialoprotein (BSP), osteocalcin (OCN) and cementum protein1 (CEMP1)) to study other possible healing pathways that encourage mineralisation and tissue repair after furcation perforation sealing in further investigations.
Also, clinical studies on patients are recommended to overcome the limitations raised by using animal models with different tissue healing rates. The healing response with epithelisation seen in the perforation of dogs’ teeth is expected to be less in humans, giving more favourable response [17] Additionally, the clinical evaluation using periodontal examinations (change in pocket depth) and three-dimensional radiographic methods (like micro-CT or CBCT analysis) is highly required to evaluate the effect of different platelet concentrates on perforation repair. Correlation between different evaluation tools for healing perforation defects is also recommended in further studies.
Conclusions
According to the findings of the present investigation, the use of two platelet concentrates, CGF and PRF with MTA, was found to be more promising than MTA repair alone in the treatment of both non-contaminated and contaminated perforations with superior outcomes in non-contaminated ones. Moreover, the current investigation findings demonstrated that CGF improved the hard and soft tissue regeneration non-significantly more than PRF.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to privacy but are available from the corresponding author on reasonable request.
Abbreviations
- MTA:
-
Mineral trioxide aggregate
- PRF:
-
Platelet-rich fibrin
- PRP:
-
Platelet-rich plasma
- CGF:
-
Concentrated growth factors
- REC:
-
Research Ethics Committee
- ARRIVE:
-
The Animal Research: Reporting in Vivo Experiments
- EDTA:
-
Ethylenediaminetetraacetic acid
- CEJ:
-
Cementoenamel junction
- OPN:
-
Osteopontin
- TRAP:
-
Tartrate-resistant acid phosphatase
- TAZ:
-
Tafazzin
References
Silva LA, Pieroni KA, Nelson-Filho P, Silva RA, HernandézGatón P, Lucisano MP (2017) Furcation perforation: periradicular tissue response to biodentine as a repair material by histopathologic and indirect immunofluorescence analyses. J Endod 43:1137–1142
Ruddle C (2001) Nonsurgical endodontic retreatment. In: Cohen S, Burns RC (eds) Pathways of the pulp, 8th edn. Mosby, St. Louis, Mo, USA, pp 875–929
Tsesis I, Fuss Z (2006) Diagnosis and treatment of accidental root perforations. Endod Topics 13:95–107
Estrela C, Pécora JD, Estrela CRA, Guedes OA, Silva BS, Soares CJ (2017) Common operative procedural errors and clinical factors associated with root canal treatment. Braz Dent J 28:179–190
Pinheiro LS, Kopper PMP, Quintana RM, Scarparo RK, Grecca FS (2021) Does MTA provide a more favourable histological response than other materials in the repair of furcal perforations? A systematic review. Int Endod J 54(12):2195–2218
Askerbeyli Örs S, Aksel H, Küçükkaya Eren S, Serper A (2019) Effect of perforation size and furcal lesion on stress distribution in mandibular molars: a finite element analysis. Int Endod J 52(3):377–384
Sinai IH (1977) Endodontic perforations: their prognosis and treatment. J Am Dent Assoc 95(1):90–95
Al-Daafas A, Al-Nazhan S (2007) Histological evaluation of contaminated furcal perforation in dogs’ teeth repaired by MTA with or without internal matrix. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 103:92–99
Estrela C, Decurcio DA, Rossi-Fedele G, Silva JA, Guedes OA, Borges AH (2018) Braz. root perforations: a review of diagnosis, prognosis and materials. Oral Res 32:73–79
Benanati FW, Roane JB, Biggs JT, Simon JH (1986) Recall evaluation of iatrogenic root perforations repaired with amalgam and guttapercha. J Endod 12:161–166
Alhadainy HA, Himel VT, Lee WB, Elbaghdady YM (1998) Use of a hydroxylapatite-based material and calcium sulfate as artificial floors to repair furcal perforations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 86:723–729
Tawfik HE, Abu-Seidab A, Hashema AA, El-Khawlania MM (2015) Treatment of experimental furcation perforations with mineral trioxide aggregate, platelet rich plasma or platelet rich fibrin in dogs’ teeth. Exp Toxicol Pathol 68:321–327
Palma PJ, Marques JA, Santos J, Falacho RI, Sequeira D, Diogo P, Caramelo F, Ramos JC, Santos JM (2020) Tooth discoloration after regenerative endodontic procedures with calcium silicate-based cements—an ex vivo study. Appl Sci 10(17):5793
Palma PJ, Marques JA, Falacho RI, Vinagre A, Santos JM, Ramos JC (2018) Does delayed restoration improve shear bond strength of different restorative protocols to calcium silicate-based cements? Materials 11(11):2216
Xavier MT, Costa AL, Caramelo FJ, Palma PJ, Ramos JC (2021) Evaluation of the interfaces between restorative and regenerative biomaterials used in vital pulp therapy. Materials 14:5055
Palma PJ, Marques JA, Antunes M et al (2021) Effect of restorative timing on shear bond strength of composite resin/calcium silicate-based cements adhesive interfaces. Clin Oral Investig 25(5):3131–3139
Cardoso M, Dos Anjos PM, Correlo V, Reis R, Paulo M, Viegas C (2018) Biodentine for furcation perforation repair: an animal study with histological, radiographic and micro-computed tomographic assessment. Iran Endod J 13(3):323–330
Zou L, Liu J, Yin S, Li W, Xie J (2008) In vitro evaluation of the sealing ability of MTA used for the repair of furcation perforations with and without the use of an internal matrix. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 105:61–65
Arshad S, Tehreem F, Rehab Khan M, Ahmed F, Marya A, Karobari MI (2021) Platelet-rich fibrin used in regenerative endodontics and dentistry: current uses, limitations, and future recommendations for application. Int J Dent 2021:4514598
Nivedhitha MS, Jacob B, Ranganath A (2020) Concentrated growth factor: a novel platelet concentrate for revascularization of immature permanent teeth-a report of two cases. Case Rep Dent 2020:1329145
Hartshorne J, Gluckman H (2016) A comprehensive clinical review of platelet rich fibrin (PRF) and its role in promoting tissue healing and regeneration in dentistry. Part III: Clinical indications of PRF in implant dentistry, periodontology, oral surgery, and regenerative endodontics. Int Dent 6(64):79
Xu F, Qiao L, Zhao Y, Chen W, Hong S, Pan J, Jiang B (2019) The potential application of concentrated growth factor in pulp regeneration: an in vitro and in vivo study. Stem Cell Res Ther 10:134–150
Choukroun J, Diss A, Simonpieri A (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101:299–303
Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J et al (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101:37–44
El Masri M, Shokry M, Attia N, Ramal A (2018) Comparison of PRF versus collagen membrane on healing of socket and bone formation (randomized split mouth design). Acta Scien Dent Scien 2:13–22
Borsani E, Bonazza V, Buffoli B, Cocchi MA, Castrezzati S, Scarì G (2015) Biological characterization and in vitro effects of human concentrated growth factor preparation: an innovative approach to tissue regeneration. Biol Med 7:1–10
Chen J, Jiang H (2020) A comprehensive review of concentrated growth factors and their novel applications in facial reconstructive and regenerative medicine. Aesthetic Plast Surg 44:1047–1057
Mijiritsky E, Assaf HD, Peleg O, Shacham M, Cerroni L, Mangani L (2021) Use of PRP, PRF and CGF in periodontal regeneration and facial rejuvenation-a narrative review. Biology (Basel) 10(4):317
Malli Sureshbabu N, Selvarasu K, Nandakumar M, Selvam D (2019) Concentrated growth factors as an ingenious biomaterial in regeneration of bony defects after periapical surgery: a report of two cases. Dent case Rep 2019:1–6
Öngöz Dede F, Bozkurt Doğan Ş, Çelen K, Çelen S, Deveci ET, Seyhan CN (2023) Comparison of the clinical efficacy of concentrated growth factor and advanced platelet-rich fibrin in the treatment of type I multiple gingival recessions: a controlled randomized clinical trial. Clin Oral Investig 27(2):645–657
Faul F, Erdfelder E, Georg Lang A, Buchner A (2007) A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191
Hall LW, Clarke KW, Trim CM (2001) Principles of sedation, analgesia and premedication. In: Saunders WB (ed) Veterinary Anesthesia, 10th edn. Harcourt, London, p 79
Silva RAB, Borges ATN, Hernandéz-Gatón P, de Queiroz AM, Arzate H, Romualdo PC, Nelson-Filho P, Silva LAB (2019) Histopathological, histoenzymological, immunohistochemical and immunofluorescence analysis of tissue response to sealing materials after furcation perforation. Int Endod J 52:1489–1500
de Sousa RM, Scarparo RK, Steier L, de Figueiredo JAP (2019) Periradicular inflammatory response, bone resorption, and cementum repair after sealing of furcation perforation with mineral trioxide aggregate (MTA Angelus™) or Biodentine™. Clin Oral Investig 23(11):4019–4027. https://doi.org/10.1007/s00784-019-02833-z
Hashem AA, Hassanien E (2008) ProRoot MTA, MTA-Angelus and IRM used to repair large furcation perforations: sealability study. J Endod 34:59–61
Dohan EDM, Pinto NR, Pereda A, Jiménez P, Del Corso M, Kang B, Nally M, Lanata N, Wang H, Quirynen M (2018) The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors, and fibrin architecture of a leukocyte-and platelet-rich fibrin (L-PRF) clot and membrane. Platelets 29:171–184
Thanasrisueb P, Wong T, Surarit R, Sompop B, Ruangsawasdi N (2019) Influence of fractionation methods on physical and biological properties of injectable platelet-rich fibrin: an exploratory study. Int J Mol Sci 20:1657–1668
Qiao J, Duan J, Zhang Y, Yi Chu, Sun C (2016) The effect of concentrated growth factors in the treatment of periodontal intrabony defects. Future Sci OA 2:4–14
Vigolo P, Zaccaria M (2010) Clinical evaluation of marginal bone level change of multiple adjacent implants restored with splinted and nonsplinted restorations: a 5-year prospective study. Int J Oral Maxillofac Implants 25(6):1189–1194
Ismail SM, Fayyad DM, Eldaharawy MH, Mohamed DA (2022) Effect of two calcium-silicate sealers and a resin sealer on collagen matrix integrity of root dentin after different treatments. An in vitro and in vivo study. Saudi Endod J 12:67–75
Sangeetha R, Uma K, Chandavarkar V (2013) Comparison of routine decalcification methods with microwave decalcification of bone and teeth. J Oral Maxillofac Pathol 17(3):386–391
Sheehan DC, Hrapchak BB (1980) Theory and practice of histotechnology, 2nd edn. The CV Mosby Company, St Louis, p 300
Basta DG, Abu Seida A, El Batouty KM, Tawfik HM (2021) Effect of combining platelet rich fibrin with synthetic bone graft on the healing of intrabony defects after apicectomy in dogs with periapical pathosis. Saudi Endod J 11:300–307
Huh SJ, Oh H, Peterson MA et al (2016) The proliferative activity of mammary epithelial cells in normal tissue predicts breast cancer risk in premenopausal women. Can Res 76(7):1926–1934
Oros J, Matsushita S, Rodriguez JL, Rodriguez F, Fernandez A (1996) Demonstration of rat CAR bacillus using a labelled streptavidin biotin (LSAB) method. J Vet Med Sci 58(12):1219–1221
Sisli SN, Yılmaz B, Özpolat Z, Gülşahı K (2021) Comparative analysis of different periapical index systems used in cone-beam computed tomography. Aust Endod J 47(3):401–407. https://doi.org/10.1111/aej.12486
Cohen JA (1960) Coefficient of agreement for nominal scales. Edu Psychol Measur 20:37–46
Hassanien EE, Abu-Seida AM, Hashem AA, Khanbash SS (2015) Histologic evaluation of furcation perforation treated with mineral trioxide aggregate and bioaggregate. Asian J Anim Sci 9:148–156
Yildirim T, Gencoglu N, Firat I, Perk C, Guzel O (2005) Histologic study of furcation perforations treated with MTA or Super EBA in dogs’ teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 100:120–124
Ivanovski S, Haase HR, Bartold PM (2001) Expression of bone matrix protein mRNAs by primary and cloned cultures of the regenerative phenotype of human periodontal fibroblasts. J Dent Res 80(7):1665–1671
Gaballah OM, Abd-Elmotelb MA, Saleh RG (2018) Distribution of osteopontin in normal dog periodontium (immunohistochemical study). Lif Sci J 15(7):321
Hayman AR, Macary P, Lehner PJ, Cox TM (2001) Tartrate-resistant acid phosphatase (Acp 5): identification in diverse human tissues and dendritic cells. Histochem Cytochem J 49:675–683
Kirstein B, Chambers TJ, Fuller K (2006) Secretion of tartrate-resistant acid phosphatase by osteoclasts correlates with resorptive behavior. Cellular Biochem J 98:1085–1094
Cau JYM, Hutter JV, Mork TO, Nicoll BK (1997) An in vitro study of furcation perforation repair using calcium phosphate cement. J Endod 23:588–593
Parirokh M, Torabinejad M (2010) Mineral trioxide aggregate: a comprehensive literature review – part III: clinical applications, drawbacks, and mechanism of action. J Endod 36:400–413
Al-Hezaimi K, Al-Hamdan K, Naghshbandi J, Oglesby S, Simon JH, Rotstein I (2005) Effect of white-colored mineral trioxide aggregate in different concentrations on Candida albicans in vitro. J Endod 31:684–668
Hartshorne J, Gluckman H (2016) A comprehensive clinical review of platelet rich fibrin (PRF) and its role in promoting tissue healing and regeneration in dentistry. Part III: clinical indications of PRF in implant dentistry, periodontology, oral surgery, and regenerative endodontics. Int Dent 6:6–79
Holzer-Geissler JC, Schwingenschuh S, Zacharias M, Einsiedler J, Kainz S, Reisenegger P, Holecek C, Hofmann E, Wolff-Winiski B, Fahrngruber H, Birngruber T, Kamolz L, Kotzbeck P (2022) The impact of prolonged inflammation on wound healing. Biomedicines 10:856–870
Dohan ED, de Peppo GM, Doglioli PG, Sammartino G (2009) Slow release of growth factors and thrombospondin-1 in Choukroun’s platelet-rich fibrin (PRF): a gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors 27:63–69
Kalén A, Wahlström O, Linder CH, Magnusson P (2008) The content of bone morphogenetic proteins in platelets varies greatly between different platelet donors. Biochem Biophys Res Commun 375:261–264
Zhang Z, Lai Q, Li Y, Xu C, Tang X, Ci J, Sun S, Xu B, Li Y (2017) Acidic pH environment induces autophagy in osteoblasts. Sci Rep 7:46161
Ford RT, Torabinejad M, Hong C, Kariyawasam S (1995) Use of mineral trioxide aggregate for repair of furcal perforations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 79:756–763
Yang JM, Yang KI, Lee KH et al (2018) Effects of platelet-rich plasma on tooth replantation in dogs: a histologic and histomorphometric analysis. J Periodontal Implant Sci 48:224–235
Shaheen D, Niazy MA, Jamil Wl E, Abu-Seida AM (2021) Pulp tissue response to platelets rich plasma, platelets rich fibrin and mineral trioxide aggregate as pulp capping materials. Azh Dent J 8(4):562–570
Dohan DM, Choukroun J, Diss A (2006) Platelet-rich fibrin (PRF): a secondgeneration platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 101:45–50
Pan J, Xu Q, Hou J et al (2019) Effect of platelet-rich fibrin on alveolar ridge preservation: a systematic review. J Am Dent Assoc 150:766–778
Li A, Yang H, Zhang J, Chen S, Wang H, Gao Y (2019) Additive effectiveness of autologous platelet-rich fibrin in the treatment of intrabony defects: a PRISMA-compliant meta-analysis. Med (Baltimore) 98:e14759
Pradeep AR, Rao NS, Agarwal E, Bajaj P, Kumari M, Naik SB (2012) Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of 3-wall intrabony defects in chronic periodontitis: a randomized controlled clinical trial. J Periodontol 83:1499–1507
Duan X, Lin Z, Lin X et al (2018) Study of platelet-rich fibrin combined with rat periodontal ligament stem cells in periodontal tissue regeneration. J Cell Mol Med 22:1047–1055
Wu CL, Lee SS, Tsai CH, Zhao JH, Chang YC (2012) Platelet rich fibrin increases cell attachment, proliferation, and collagen related protein expression of human osteoblasts. Aust Dent J 57:207–212
Nguyen TH, Palankar R, Bui VC (2016) Rupture forces among human blood platelets at different degrees of activation. Sci Rep 6:1–12
Wang HL, Boyapati L (2006) PASS” principles for predictable bone regeneration. Implant Dent 15:8–17
Nityasri Aromal S, Pradeep KY, Kalaivani V, Raja P (2018) Role of CGF (concentrated growth factor) in periodontal regeneration. J Dent Health Oral Disord Ther 9:350–352
Li W, Wang F, Dong F, Zhang Z, Song P, Chen H, Wang J (2021) CGF membrane promotes periodontal tissue regeneration mediated by hUCMSCs through upregulating TAZ and osteogenic differentiation genes. Stem Cells Int 4(2021):6644366
Song JS, Mante FK, Romanow WJ, Kim S (2006) Chemical analysis of powder and set forms of Portland cement, gray ProRoot MTA, white ProRoot MTA, and gray MTA-Angelus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 102:809–815
Shokouhinejad N, Nekoofar MH, Pirmoazen S, Shamshiri AR, Dummer PM (2016) Evaluation and comparison of occurrence of tooth discoloration after the application of various calcium silicate-based cements: an ex vivo study. J Endod 42(1):140–144
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contribute to this research: Dr. DAM and Prof. RMT: research designing, animal study, collecting and analysis of the data and writing of the scientific manuscript. Dr. SAA and Prof. RHM: analysis of the data and writing and review of the scientific manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Research Ethics Committee (REC), Faculty of Dentistry, Suez Canal University, Egypt. Protocol No:335/2021. All methods were carried out in accordance with relevant Institutional guidelines and regulations for animal care and use. Furthermore, all methods were reported in accordance with the Animal Research Reporting in vivo Experiments requirements (ARRIVE) guidelines.
Consent for publication
Not applicable according to the ethical committee at Faculty of Dentistry, Suez Canal University, Egypt.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohamed, D.AA., Abdelwahab, S.A., Mahmoud, R.H. et al. Radiographic and immuno-histochemical evaluation of root perforation repair using MTA with or without platelet-rich fibrin or concentrated growth factors as an internal matrix in dog’s teeth: in vivo animal study. Clin Oral Invest 27, 5103–5119 (2023). https://doi.org/10.1007/s00784-023-05131-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-023-05131-x