Abstract
Objectives
The aim of this retrospective case series was to evaluate the efficacy and volume stability of a customized allogeneic bone block (CABB) for the hard tissue reconstruction of severely atrophied anterior maxillary ridges.
Materials and methods
Hard tissue alterations between baseline (T1), 2-month follow-up (T2), and 6-month follow-up (T3) cone-beam computed tomography scans were evaluated with semi-automatic segmentation. Following automatic spatial alignment of the datasets, 3D subtraction analysis was performed. The volume stability of the inserted allogeneic bone block was determined on the basis of the ratio of the T3 and T2 hard tissue volumes.
Results
The newly formed hard tissue volume at T2 averaged at of 0.75 cm3 ± 0.57 cm3, whereas at T3, an average of 0.52 cm3 ± 0.42 cm3 volumetric hard tissue gain could be detected. The T3/T2 ratio was found to be 67.83% ± 18.72% on average. The dice similarity coefficient between the T2 and T3 hard tissue models averaged at 0.73 ± 0.15.
Conclusions
Cancellous CABBs are a reliable option for the reconstruction of severely atrophied alveolar ridges. The resorption rates of these grafts are similar to those found in the literature; however, with precise manufacturing and proper intraoperative flap management, the resorption rates may be reduced.
Clinical relevance
With precise knowledge of the resorption patterns, the shape of blocks can be altered in the future to compensate for the volumetric loss.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of surgical and prosthetic solutions in implant dentistry has led to a state where there are multiple scientifically proven treatment options for almost any clinical scenario [1]. Implant-born prosthetic solutions are even feasible at edentulous areas presenting severe alveolar hard tissue impairment. Different surgical approaches can be utilized to reestablish sufficient hard tissue mass necessary for implant placement. Surgical techniques include distraction osteogenesis, ridge splitting, guided bone regeneration (GBR), and onlay grafting [2, 3]. Onlay grafting utilizing intra- or extraoral block grafts and staged GBR yield similar results both in terms of the complication rate and implant survival rate [4]. In cases of severely atrophied alveolar ridges, intraoral autogenous bone blocks may not present sufficient volumes; therefore, large bone grafts must be harvested from extraoral donor sites (e.g., iliac crest or calvaria). The necessity of hospitalization, unpredictable premature graft resorption, and donor site morbidity are the most common complications of OG utilizing extraoral donor sites [5]. Various studies have reported different graft resorption rates [6,7,8,9,10,11,12,13,14,15,16,17]. Contradictory data are attributed to differences in the observation period, type of reconstruction, use of provisional dentures, and location of the donor site among studies. Despite literature inconsistencies, different authors agree that (i) the greatest bone resorption occurs in the first year after alveolar ridge augmentation; (ii) there are significant differences between bone blocks harvested from different donor sites (calvarial grafts yield lower resorption rates than do iliac grafts); (iii) grafts should be oversized to compensate for the resorption; (iv) the use of corticocancellous blocks is advised; and (v) removable dentures should be avoided at the surgical area, as they may cause complete resorption of the graft [16].
As an alternative to autogenous block grafts, allogeneic grafts can be used for the reconstruction of highly atrophied alveolar ridges. Thereby, the preparation of a second surgical site and the occurrence of a donor site complication can be completely avoided, reducing the invasiveness of the procedure. In the systematic review by Monje and co-workers [18] summarizing data of 15 articles, the mean resorption rate of allogenous bone blocks was found to be lower (mean, 21.7%) than that of autogenous bone blocks. Allogeneic bone blocks are hence favored not only by patients owing to reduced invasiveness and the non-necessity of hospitalization but also by clinicians owing to the reduced amount of unwanted graft resorption. Wang and co-workers [19] recently compared the efficacy between customized allogeneic bone blocks (CABBs) and autogenous bone blocks for alveolar ridge augmentation. They reported less bone resorption with customized allogeneic bone grafts than with autogenous bone grafts and emphasized the importance of three-dimensional (3D) technology for an elaborative preoperative planning. Based on preoperative cone-beam computed tomography (CBCT) scans, CABBs are created using computer-aided design/computer-aided manufacturing (CAD/CAM) technologies. With CAD/CAM, high-accuracy CABBs can be manufactured; therefore, intraoperative manual adjustments are largely unnecessary, reducing the duration of the procedure, risk of graft contamination, and pressure applied by soft tissues owing to the more anatomical shape [20, 21]. In addition, the space between the grafts and recipient alveolar ridges is minimal, resulting in a larger contact surface, which is an ideal support for graft revascularization and rapid integration [22,23,24]. This is of particular importance for complex bone defect morphologies [24].
To date, the literature on the resorption patterns and 3D morphological alterations of CABBs following hard tissue reconstruction is limited. Hence, the aim of this retrospective case series was to evaluate the efficacy and volume stability of CABBs for the hard tissue reconstruction of severely atrophied anterior maxillary ridges.
Materials and methods
Null hypothesis
Our hypothesis was that in terms of volumetric heart tissue gain and resorption rate, similar results can be achieved with the application of CABBs achieved as with autogenous bone blocks.
Study design
In this retrospective study, 23 patients presenting severe combined horizontal and vertical alveolar ridge defects in the anterior maxillary region treated with a cancellous CABB (maxgraft bonebuilder, botiss GmbH, Zossen, Germany) were evaluated. The participants were treated at the Back & Blume private dental office with a customized CAD/CAM allogeneic bone block between 2017 and 2020. The study was conducted in full accordance with the revised Declaration of Helsinki (2013) [25] and was approved by our local ethics committee (Ethical Committee of Ludwig-Maximilians-University Munich, Germany; approval number: 18-898). A signed written informed consent was acquired from all participants.
Patient selection
The inclusion criteria were as follows: (i) absence of general medical conditions ( previous irradiation therapy in the head and neck region within the last 2 years, uncontrolled diabetes, systemic steroid treatment, or systemic bisphosphonate treatment); (ii) non-smoking status; (iii) full mouth plaque score of ≤25% [26]; (iv) full mouth bleeding score of ≤25% [27]; and (v) healed alveolar ridge defects unfitted for implant placement with or without simultaneous hard tissue augmentation.
The exclusion criteria were as follows: (i) prolonged antibiotic/anti-inflammatory therapy prior to surgery, (ii) substance abuse, (iii) pregnancy or lactation, (iv) under 18 years of age, and (v) post-extraction defects and tooth extraction within 3 months prior to hard tissue augmentation (Figs. 1 and 2A).
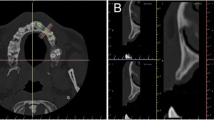
Baseline radiographic images. A 3D reconstruction with semi-automatic segmentation of an alveolar ridge defect at a multiple-tooth gap. B 3D reconstruction with semi-automatic segmentation of an alveolar ridge defect at a single-tooth gap. C Sagittal view of the baseline ridge dimensions at a multiple-tooth gap. D Sagittal view of the baseline ridge dimensions at a single-tooth gap
Data acquisition and surgical planning
CBCT scans were obtained at three different time points: baseline (T1), 2-month follow-up (T2), and 6-month follow-up (T3). They were taken at the following parameters using an I-CAT FLX CBCT machine (KaVo Dental GmbH, Bieberach an der Riß, Germany): (i) voxel size (0.250 μm), (ii) X-ray tube current (4–7 mA), (iii) voltage (120 kVp), and (iv) field of view (16.5 × 13.5 cm) [28]. Digital Imaging and Communications in Medicine images of the baseline CBCT datasets were imported into a digital planning software (coDiagnostiX, version 10.2.0.15659, Dental Wings Inc., Montreal, Canada) for the assessment of the baseline alveolar ridge defect morphology and planning of the CABB. The baseline alveolar ridge defects were classified at each implantation site according to the HVC ridge deficiency classification [29]. Following planning, a CABB was manufactured from the femoral heads of living donors who underwent arthroplastic surgery (maxgraft bonebuilder, botiss biomaterials GmbH, Berlin, Germany). For the prevention of pressure and eventual graft resorption caused by the protruding screw head, a countersink was incorporated into the design of the blocks.
Surgical treatment protocol
Flap elevation
The bone augmentation procedures were conducted under general anesthesia. The extent of the edentulous area did not affect the surgical modality. Flap preparation on the buccal aspect was conducted on the basis of the semi-pillar incision design introduced by our group [22]. A horizontal incision was created on the buccal aspect within the mobile mucosa approximately 20 mm apically from the midcrestal line. Thereafter, a single vertical releasing incision was created at the distal aspect of the surgical area. A unilateral full-thickness mucoperiosteal flap was subsequently elevated on the buccal aspect, while the keratinized mucosa on the crestal and palatal aspects remained attached to the underlying bone (Fig. 2B).
Cancellous allogeneic bone block fixation
Prior to block positioning, the cortical layer at the augmented site was perforated using a diamond bur to induce bleeding for an enhanced vascularization of the graft. Following the hydration of the graft, the allogeneic bone block was placed onto the recipient site without any further adjustments. Block fixation was conducted using titanium osteosynthesis screws (Medartis AG, Basel, Switzerland) (Fig. 2C). Thereafter, the area was covered with a long-term resorbable porcine pericardium membrane (Jason membrane, botiss biomaterials GmbH, Zossen, Germany) and was fixated with titanium pins (Fig. 2D) for additional barrier function. Tension-free wound closure was achieved with single interrupted sutures utilizing 4-0 and 5-0 resorbable suturing materials (Vicryl Rapide, Ethicon, Raritan, New Jersey, USA). The sutures were removed after 14 days.
Re-entry procedure and implant placement
Following a 6-month healing period, guided implant placement was planned on the T3 CBCT scan. Direct evaluation of the reconstructed alveolar ridge, removal of the block fixation screw, and placement of a dental implant were performed during a re-entry procedure. Further hard tissue augmentation was not necessary in any of the cases (Fig. 3).
Radiographic evaluation of hard tissue changes
Segmentation—3D model acquisition
An open-source medical image processing software platform (3D Slicer, www.slicer.org) [30] was utilized to reconstruct the T1, T2, and T3 CBCT images as 3D virtual models. A region-growing segmentation method was utilized to acquire 3D models of the maxillary alveolar bone. Two separate labels were generated: one for the maxillary alveolar bone and one for the background (including teeth). Seed points were placed on the planar images of the CBCT dataset both labels; thereafter, labels were generated automatically for the maxillary alveolar bone and the background activating the region-growing function (Fig. 4A and B). On T3 and T2 CBCT scans, the heads of the osteosynthesis screws were excluded from the label representing the bone volume; however, screw bodies were included. The reason is that the screw channel after removal of the screw can be considered as very small containing defect that has very limited impact on the outcomes.
Presentation of the semi-automatic segmentation method. A Alveolar bone and background input labels generated on the axial view of the CBCT scan 3D model acquired via semi-automatic segmentation (green, alveolar bone; red, background). B 3D model of the alveolar bone following region-growing segmentation. C Labels generated for each tooth separately serves as the input of the watershed segmentation. D 3D surface representation of label maps following the application of the watershed segmentation method
Subsequently, teeth were segmented separately utilizing a watershed image segmentation method. Gray value intensity range of the teeth was determined, and seed points were drawn inside each individual tooth. Subsequently, the watershed segmentation tool was utilized to automatically generate a label for all teeth simultaneously (Fig. 4C and D) [31].
Evaluation of radiographic hard tissue changes
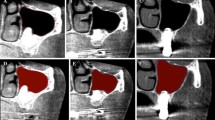
Following image segmentation of all CBCT datasets, automatic voxel intensity-based registration was performed [32] to align the three CBCT scans in the virtual space. Thereafter, hard tissue changes between the three time points were determined by subtracting the segmented 3D models from one another by the means of logical operators. For example, to assess the volumetric gain between T2 and T1 time points, the T1 CBCT model was subtracted from the T2 CBCT model. Subsequently, 3D models of the new hard tissues at both T2 and T3 were generated (Fig. 5).
The volume stability of the inserted allogeneic bone blocks was determined on the basis of the T3/T2 ratio (%) in both methods. Simultaneously, the dice similarity coefficient (DSC) was calculated to determine the spatial overlap between the models of newly formed hard tissue at T2 and T3 time points. The DSC was used to reflect how well the implanted CABB retained its original shape (Fig. 6).
Besides utilizing the aforementioned semi-automatic segmentation method, a global thresholding method was also utilized to evaluate outcomes. Results are described in the supplementary file.
Linear measurements at future implantation sites were performed to validate the vertical and horizontal dimensions of the edentulous crest at each corresponding time point. Linear measurement method and results are summarized in the supplementary file.
Outcome measures
The primary outcome of the study was the volumetric hard tissue change between T1, T2, and T3 time points. The secondary outcomes were (i) volumetric stability of the CABB determined by the T3/T2 ratio, (ii) morphological stability expressed by the DSC value, and (iii) assessment of linear hard tissue dimensions at T1, T2, and T3 time points.
Sample size calculation
Sample size was calculated by the means of power analysis using the G*Power statistical power analysis software (Heinrich Heine Universität Düsseldorf, Düsseldorf, Germany). Mean and standard deviation values of T2 and T3 volumetric gain values were determined as input parameters. The effect size (Cohen’s dz) was determined to be 0.55, and with α value of 0.05 and a power of 0.80, the total sample size was calculated to be 23.
Statistical analysis
Descriptive statistics were used to describe the overall hard tissue changes among all participants. The overall changes were expressed as means ± standard deviations. The normality of the examined variables was checked with the Shapiro-Wilk test. Levene’s test checked the homogeneity of the variances. Data were found to be non-normally distributed, and variance homogeneity was also broken. The continuous variables between subgroups were compared with non-parametric statistics. The Wilcoxon matched pairs signed rank test was utilized to evaluate statistical differences for each variable at different time points. Correlation between the T3/T2 ratio and the DSC values was determined by Spearman rank order correlation test. The analysis was two sided with a significance level of α = 0.05. The statistical analysis was performed using the IBM® SPSS® 28.0 (IBM Corporation Armonk, NY, USA) program package.
Results
Patient demographics and baseline defect characteristics
Among the 23 participants, 14 were men, and 9 were women. The mean age was 45.48 ± 12.52 years. Single-tooth gaps were present in 13 participants, whereas 10 participants presented a multiple-tooth gap (two-tooth gap, 6 patients; three-tooth gap, 3 patients; and four-tooth gap, 2 patients). Linear measurements were taken at 40 future implantation sites.
According to the HVC ridge deficiency classification, 27 defects were classified as large horizontal defects, 6 as large combination defects, 6 as medium combination defects, and 1 as a medium horizontal defect. Data are summarized in Table 1.
Volumetric hard tissue changes
At T2, an average of 0.75 cm3 ± 0.57 cm3 volumetric hard tissue gain was detected, with a median value of 0.49 cm3. At T3, an average of 0.52 cm3 ± 0.42 cm3 volumetric hard tissue gain could be detected with a median value of 0.37 cm3. As the result of the statistical analysis, a statistically significant volumetric hard tissue resorption was found between T2 and T3 (p < 0.05). Data are summarized in Table 2.
Volumetric and morphological stability of CABBs
The average volume stability of the CABBs determined by the T3/T2 ratio was found to be 67.83% ± 18.72% on average with a median value of 72.46%. The DSC between the T2 and T3 hard tissue models averaged at 0.73 ± 0.15 with a median value of 0.77. High level of correlation between the T3/T2 ratio and the DSC value was found (Spearman’s correlation coefficient, 0.93, p < 0.05). These data are summarized in Table 3. As it is visible on the colormap models, hard tissue resorption is most expressed on the buccal/crestal aspect of the block (Fig. 5).
Discussion
In the current study, 23 advanced alveolar ridge deficiencies in the upper anterior region were reconstructed using CABBs. Volumetric radiographic evaluation was performed by comparing segmented 3D CBCT datasets in different time points. The primary outcome of the study was to evaluate the volumetric gain achieved over the course of 6-month healing period. Additionally, the current paper focused on assessing the volumetric and morphological stability of the implanted CABB between T2 and T3 time points. At T2, the average new hard tissue formation was found to be 0.75 cm3 ± 0.57 cm3 which reduced to 0.52 cm3 ± 0.42 cm3 at T3 resulting in an approximately 32% volumetric hard tissue This resorption rate is similar to previously reported data on cancellous allogeneic bone blocks—approximately 29% [33]. To our knowledge, no data was reported on the spatial overlap of the inserted bone block between two different time points. This metric, expressed by the DSC value, was found to be 0.73 ± 0.15 on average. The DSC values showed high levels of correlation to the T3/T2 ratio indicating the volumetric stability. Resorption patterns are difficult to express numerically, even though from a clinical point of view this observation is very important, since the interpretation of these results may lead to the enhancement of the surgical techniques and the development of new surgical materials. Similarly to the volumetric data, a significant linear vertical and horizontal hard tissue resorption was detected between T2 and T3. In the study by Wang and co-workers, horizontal resorption of the corticocancellous allogeneic graft averaged at 2.28 mm ± 1.14 mm, and vertical hard tissue dimension loss averaged at 1.77 mm ± 0.95 mm. Whereas in the current investigation, the horizontal alveolar ridge dimensions were reduced by about 1.4–1.5 mm, the vertical alveolar ridge dimensions at the treated sites were reduced by about 0.5–0.7 mm. The implanted cancellous CABBs herein presented similar or less dimensional loss than did those in the studies by Tresguerres et al. and Wang et al. [19, 33]. Within the limitations of the current study, the reasons for the more favorable outcomes would be difficult to determine. However, it can be emphasized that the limited flap elevation and the semi-pillar incision facilitated tension-free covering of the grafts. The high-precision CAD/CAM of the CABBs may have also contributed to the seemingly better results in this study [22]. The current volumetric and graft stability data are in line with the literature.
The volumetric changes were evaluated using two methods, although these methods showed a high correlation with. Difference between the two methods could be attributed to the fact that the algorithm of global thresholding segmentation automatically labels voxels that fall within the threshold range. Contrary to the semi-automatic segmentation, this method does not recognize anatomical features and artifacts on CBCT scans. Meanwhile, during semi-automatic segmentation, the input data for region-growing and watershed algorithms are generated manually by a human. Nonetheless, both methods were found to be feasible for the volumetric evaluation of hard tissue changes, although utilizing 3D Slicer served as a much more elaborative approach.
The greatest limitation of the current study is the relatively large diversity of the baseline defect morphologies. Although all cases presented a horizontal alveolar ridge deficiency, the baseline horizontal ridge dimensions varied greatly (1.49–6.43 mm). Simultaneously, not all future implantation sites presented a vertical dimension loss, with only 12 being categorized as combination defects according to the HVC classification.
Conclusions
The current findings support previous literature data. Therefore, it can be concluded that cancellous CAD/CAM allogeneic bone blocks can be reliably utilized for the reconstruction of severely atrophied alveolar ridges. The resorption rates of these grafts are similar to those found in the literature; however, with precise manufacturing and proper intraoperative flap management, the rates may be reduced. Although morphological alterations are difficult to express numerically, these findings could contribute to the future development of surgical techniques and regenerative materials.
References
Buser D, Sennerby L, de Bruyn H (2017) Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontol 73(1):7–21. https://doi.org/10.1111/prd.12185
Plonka AB, Urban IA, Wang HL (2018) Decision tree for vertical ridge augmentation. Int J Periodontics Restorative Dent 38:269–275. https://doi.org/10.11607/prd.3280
Fu JH, Wang HL (2011) Horizontal bone augmentation: the decision tree. Int J Periodontics Restorative Dent 31:429–436
Milinkovic I, Cordaro L (2014) Are there specific indications for the different alveolar bone augmentation procedures for implant placement? A systematic review. Int J Oral Maxillofac Surg 43:606–625. https://doi.org/10.1016/j.ijom.2013.12.004
Nkenke E, Neukam FW (2014) Autogenous bone harvesting and grafting in advanced jaw resorption: morbidity, resorption and implant survival. Eur J Oral Implantol 7(Suppl 2):S203–S217
Becktor JP, Eckert SE, Isaksson S, Keller EE (2002) The influence of mandibular dentition on implant failures in bone-grafted edentulous maxillae. Int J Oral Maxillofac Implants 17:69–77
Chiapasco M, Abati S, Romeo E, Vogel G (1999) Clinical outcome of autogenous bone blocks or guided bone regeneration with e-PTFE membranes for the reconstruction of narrow edentulous ridges. Clin Oral Implants Res 10:278–288. https://doi.org/10.1034/j.1600-0501.1999.100404.x
Donovan MG, Dickerson NC, Hanson LJ, Gustafson RB (1994) Maxillary and mandibular reconstruction using calvarial bone grafts and Branemark implants: a preliminary report. J Oral Maxillofac Surg 52:588–594. https://doi.org/10.1016/0278-2391(94)90096-5
Esposito M, Worthington HV, Thomsen P, Coulthard P (2004) Interventions for replacing missing teeth: different times for loading dental implants. Cochrane Database Syst Rev:Cd003878 3:4. https://doi.org/10.1002/14651858.CD003878.pub2
Jemt T, Lekholm U (2003) Measurements of buccal tissue volumes at single-implant restorations after local bone grafting in maxillas: a 3-year clinical prospective study case series. Clin Implant Dent Relat Res 5:63–70. https://doi.org/10.1111/j.1708-8208.2003.tb00185.x
Lekholm U, Wannfors K, Isaksson S, Adielsson B (1999) Oral implants in combination with bone grafts. A 3-year retrospective multicenter study using the Brånemark implant system. Int J Oral Maxillofac Surg 28:181–187
Lundgren S, Nyström E, Nilson H, Gunne J, Lindhagen O (1997) Bone grafting to the maxillary sinuses, nasal floor and anterior maxilla in the atrophic edentulous maxilla. A two-stage technique. Int J Oral Maxillofac Surg 26:428–434. https://doi.org/10.1016/s0901-5027(97)80007-0
Nyström E, Ahlqvist J, Gunne J, Kahnberg KE (2004) 10-year follow-up of onlay bone grafts and implants in severely resorbed maxillae. Int J Oral Maxillofac Surg 33:258–262. https://doi.org/10.1006/ijom.2003.0512
Schliephake H, Neukam FW, Wichmann M (1997) Survival analysis of endosseous implants in bone grafts used for the treatment of severe alveolar ridge atrophy. J Oral Maxillofac Surg 55(1227-33):1233–1234. https://doi.org/10.1016/s0278-2391(97)90173-7
van Steenberghe D, Naert I, Bossuyt M, De Mars G, Calberson L, Ghyselen J, Brånemark PI (1997) The rehabilitation of the severely resorbed maxilla by simultaneous placement of autogenous bone grafts and implants: a 10-year evaluation. Clin Oral Investig 1:102–108. https://doi.org/10.1007/s007840050020
Chiapasco M, Zaniboni M (2011) Failures in jaw reconstructive surgery with autogenous onlay bone grafts for pre-implant purposes: incidence, prevention and management of complications. Oral Maxillofac Surg Clin North Am 23:1–15. https://doi.org/10.1016/j.coms.2010.10.009
Widmark G, Andersson B, Ivanoff CJ (1997) Mandibular bone graft in the anterior maxilla for single-tooth implants. Presentation of surgical method. Int J Oral Maxillofac Surg 26:106–109. https://doi.org/10.1016/s0901-5027(05)80827-6
Monje A, Pikos MA, Chan HL, Suarez F, Gargallo-Albiol J, Hernández-Alfaro F, Galindo-Moreno P, Wang HL (2014) On the feasibility of utilizing allogeneic bone blocks for atrophic maxillary augmentation. Biomed Res Int 2014:814578. https://doi.org/10.1155/2014/814578
Wang M, Li Y, Su Z, Mo A (2023) Clinical and radiographic outcomes of customized allogeneic bone block versus autogenous bone block for ridge augmentation: 6 month results of a randomized controlled clinical trial. J Clin Periodontol 50:22–35. https://doi.org/10.1111/jcpe.13714
Jacotti M, Wang HL, Fu JH, Zamboni G, Bernardello F (2012) Ridge augmentation with mineralized block allografts: clinical and histological evaluation of 8 cases treated with the 3-dimensional block technique. Implant Dent 21:444–448. https://doi.org/10.1097/ID.0b013e31826f7a67
Jacotti M, Barausse C, Felice P (2014) Posterior atrophic mandible rehabilitation with onlay allograft created with CAD-CAM procedure: a case report. Implant Dent 23:22–28. https://doi.org/10.1097/id.0000000000000023
Blume O, Hoffmann L, Donkiewicz P, Wenisch S, Back M, Franke J, Schnettler R, Barbeck M (2017) Treatment of severely resorbed maxilla due to peri-implantitis by guided bone regeneration using a customized allogenic bone block: a case report. Materials (Basel) 10:1213. https://doi.org/10.3390/ma10101213
Schlee M, Rothamel D (2013) Ridge augmentation using customized allogenic bone blocks: proof of concept and histological findings. Implant Dent 22:212–218. https://doi.org/10.1097/ID.0b013e3182885fa1
Eskow AJ, Mealey BL (2014) Evaluation of healing following tooth extraction with ridge preservation using cortical versus cancellous freeze-dried bone allograft. J Periodontol 85:514–524. https://doi.org/10.1902/jop.2013.130178
Emanuel EJ (2013) Reconsidering the Declaration of Helsinki. Lancet 381:1532–1533. https://doi.org/10.1016/s0140-6736(13)60970-8
Lang NP, Cumming BR, Löe HA (1977) Oral hygiene and gingival health in Danish dental students and faculty. Community Dent Oral Epidemiol 5:237–242. https://doi.org/10.1111/j.1600-0528.1977.tb01647.x
Lang NP, Joss A, Orsanic T, Gusberti FA, Siegrist BE (1986) Bleeding on probing. A predictor for the progression of periodontal disease? J Clin Periodontol 13:590–596. https://doi.org/10.1111/j.1600-051x.1986.tb00852.x
Jacobs R, Salmon B, Codari M, Hassan B, Bornstein MM (2018) Cone beam computed tomography in implant dentistry: recommendations for clinical use. BMC Oral Health 18:88. https://doi.org/10.1186/s12903-018-0523-5
Wang HL, Al-Shammari K (2002) HVC ridge deficiency classification: a therapeutically oriented classification. Int J Periodontics Restorative Dent 22:335–343
Pinter C, Lasso A, Fichtinger G (2019) Polymorph segmentation representation for medical image computing. Comput Methods Programs Biomed 171:19–26. https://doi.org/10.1016/j.cmpb.2019.02.011
Palkovics D, Solyom E, Molnar B, Pinter C, Windisch P (2021) Digital hybrid model preparation for virtual planning of reconstructive dentoalveolar surgical procedures. J Vis Exp 174:e62743. https://doi.org/10.3791/62743
Klein S, Staring M, Murphy K, Viergever MA, Pluim JP (2010) elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging 29:196–205. https://doi.org/10.1109/tmi.2009.2035616
Tresguerres FGF, Cortes ARG, Hernandez Vallejo G, Cabrejos-Azama J, Tamimi F, Torres J (2019) Clinical and radiographic outcomes of allogeneic block grafts for maxillary lateral ridge augmentation: a randomized clinical trial. Clin Implant Dent Relat Res 21:1087–1098. https://doi.org/10.1111/cid.12834
Funding
Open access funding provided by Semmelweis University.
Author information
Authors and Affiliations
Contributions
O.B. performed surgical treatment and 3D evaluation and reviewed the manuscript. M.B. performed surgical treatment and reviewed manuscript. E.D. did statistical analysis and reviewed the manuscript. D.P. performed volumetric evaluation, did statistical analysis, prepared all figures, and wrote the main manuscript text. P.W. reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in full accordance with the revised Declaration of Helsinki (2013) [25] and was approved by the local ethical committee (Ethical Committee Ludwig-Maximilians University Munich, Germany; approval number: 18-898). A signed written informed consent was acquired from all the participants.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1:
Supplementary File. Efficacy and volume stability of a customized allogeneic bone block for the reconstruction of advanced alveolar ridge deficiencies at the anterior maxillary region: A retrospective radiographic evaluation
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Blume, O., Back, M., Dinya, E. et al. Efficacy and volume stability of a customized allogeneic bone block for the reconstruction of advanced alveolar ridge deficiencies at the anterior maxillary region: a retrospective radiographic evaluation. Clin Oral Invest 27, 3927–3935 (2023). https://doi.org/10.1007/s00784-023-05015-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-023-05015-0