Abstract
Objectives
Ventilator-associated pneumonia (VAP) is one of the most common nosocomial infections in intensive care units (ICUs), and the use of mouthwash is the most widely used method to prevent its incidence. The aim of this study was to investigate effect of clove mouthwash on the incidence of VAP in the ICU.
Materials and methods
This comparative, randomized, triple-blind, clinical trial was conducted on 168 eligible ICU patients at Kosar Hospital in Semnan, Iran, during 2021–2022, who were divided into intervention and control groups using random blocks. The intervention group received clove extract mouthwash at 6.66% concentration, and the control group received chlorhexidine 0.2% twice a day for 5 days (routine care). Data were collected using a demographic questionnaire, and disease severity was measured based on the Acute Physiology and Chronic Health Evaluation II (APACHE II) score, oral health status was examined using the Beck Oral Assessment Scale (BOAS), and VAP diagnosis was made based on the Modified Clinical Pulmonary Infection Score (MCPIS).
Results
Before the intervention, there was no significant difference in disease severity (p = 0.412) and oral health status (p = 0.239) between the patients in the two groups. After the intervention, 20.2% of the patients in the intervention group and 41.7% of those in the control group acquired VAP. The risk of VAP was 2.06 times higher in the control group than in the intervention group (p = 0.005, 95% CI: 1.26–3.37, RR = 2.06), but the severity of VAP did not differ significantly between the patients in the two groups (p = 0.557).
Conclusion
The findings showed that clove mouthwash reduces the incidence of VAP significantly.
Clinical relevance
Clove mouthwash can be used as a simple and low-cost method to prevent VAP in ICU patients.
Similar content being viewed by others
Introduction
Respiratory infection is the most frequent nosocomial infection found in intensive care units (ICUs) [1]. Most patients who are hospitalized in the ICU need mechanical ventilation (MV) due to advanced respiratory problems [2]. MV is performed to maintain open airways, prevent aspiration, and increase oxygenation. These supportive interventions are essential to critical care and are regarded as the benchmarks for managing the airways and ventilation [3]. One of the most common side effects of MV is ventilator-associated pneumonia (VAP), which is among the most frequently occurring nosocomial infections and accounts for 25% of ICU-acquired infections [4, 5].
VAP is a form of hospital-associated pneumonia that occurs over 48 h after endotracheal intubation. VAP is diagnosed in 15% of ICU patients and is associated with a significant mortality rate of 20–30% [6]. It has been reported that 5–40% of patients undergoing invasive MV for more than 2 days acquire VAP [7]. VAP is a relatively common nosocomial infection developed in critically ill patients, with a pooled incidence ranging from 23.8% [8] to 36.0% [9] according to recent systematic reviews. VAP remains a major contributor to hospital-acquired infections in Asia [10]. The incidence rate of VAP in MV patients is 32.9% in Iran [11].
The artificial airway created to establish MV changes the mucosal defense function of the natural airway, and subsequently, bacteria enter the lower respiratory tract directly or through the gap between the wall of the tracheal tube and the airway [12]. The first step in the pathogenesis of infection involves the upper respiratory tract with potentially pathogenic organisms, including Pseudomonas and Escherichia coli. The entry of these microbes through the endotracheal tube or leakage around the cuff allows them to enter the lower respiratory tract, which, along with the host’s immunodeficiency, leads to active clinical infection. The common risk of developing VAP in the first week reaches its peak due to endotracheal intubation [6]. Aspiration of gastrointestinal microbes also increases the risk of VAP in MV patients [2]. Endotracheal intubation, the accumulation of secretions behind the tracheal tube cuff, the mouth left open and dry mouth, impairment of the cough reflex, inability to remove secretions through the pharynx and mouth, and unsatisfactory oral care may lead to VAP [13].
Since VAP increases ICU length of stay, MV duration, treatment costs, and mortality [4, 12], it is extremely important to pay close attention to VAP prevention measures [6].
A review of literature shows that unsatisfactory oral care causes mucosal dryness, decrease in salivary flow, colonization of pathogenic bacteria in the mouth and oropharynx, and acquisition of VAP [2]. In ICU patients, some pathologic changes develop in the oral cavity, such as oral mucosal lesions, periodontal disease relief, dry lips and dry mucous membranes, fungus infections, and increased biofilm on the oral surface. The majority of VAP cases are caused mainly by the micro-aspiration of oropharynx colonization agents [14, 15]. MV patients become incapable of chewing and are required to keep their mouth open, which reduces saliva flow and causes dryness of the mucous membranes [16]. According to Ademar Takahama et al. (2021), the development of VAP may be indicated by the presence of a coated tongue and oral bleeding during ICU admission [17]. There are millions of bacteria in the mouth. Although we cannot sterilize the oral environment completely, we can minimize the presence of bacteria substantially in order to prevent dry mouth and, more importantly, the accumulation of bacteria in the mouth [18].
Oral care is an essential strategy to reduce the accumulation of oropharyngeal bacteria and the presence of VAP-causing bacteria [19] and is thus one of the main strategies for VAP prevention [20]. The incidence of VAP in the ICU may be reduced by maintaining good oral hygiene, particularly with regard to the tongue, prior to intubation [17]. In the care of the critically ill, oral health is a crucial factor. According to research findings, using mouthwash containing 3% hydrogen peroxide significantly decreases the incidence of VAP compared to using mouthwash containing 0.9% normal saline [11].
Oral hygiene care using mouthwash, gel, swab, and toothbrush or a combination of them along with secretion suction may reduce the risk of VAP in patients [2]. Mouthwash is a very important strategy to reduce the colonization of oropharyngeal bacteria that cause VAP [19]. Nonetheless, there is still no preferred protocol for the prevention of VAP [21].
Dental and mucosal cleansing with chlorhexidine may decrease the risk of VAP [2]. Chlorhexidine (CHX) is a cationic biguanide that binds to the bacterial cell walls, impairing and even perforating phospholipid membranes [22]. Depending on the product’s concentration, the effect may be bacteriostatic or bactericidal [23]. In patients receiving MV, using chlorhexidine mouthwash for oral hygiene reduces the risk of VAP [2]. Chlorhexidine works to reduce bacterial colonization in the oral cavity [2, 24]. The effectiveness of any mouthwash is restricted by the biofilm that exists on the surface of the teeth [2]. Prior mechanical disruption of dental biofilms through toothbrushing improves the effect of chlorhexidine and prevents VAP [2, 25]. Chlorhexidine has lipophilic groups that can bind to bacterial cell walls and change their osmotic balance [22]. This effect inhibits bacterial growth and can even prevent death, and its mechanism of action depends on the concentration of the substance [23]. Chlorhexidine mouthwash or gel decreases the incidence of VAP compared to placebo or routine care from 26 to about 18% (RR: 0.67, 95% confidence intervals (CI): 0.47 to 0.97; p = 0.03; I2 = 66%) [2].
The clinical use of chlorhexidine gluconate dates back to the 1950s. As an antiseptic mouthwash, chlorhexidine has antimicrobial effects on the bacteria, fungi, and viruses responsible for a variety of oral diseases. All the anti-bacterial effects of chlorhexidine in vitro are attributed to altered cell membrane permeability [26]. Chlorhexidine has a bacteriostatic effect at low concentrations (0.02–0.06%) and causes the displacement of Ca2 + and Mg2 + and loss of K + from the cell wall [26, 27]. Chlorhexidine has a bactericidal effect (cell lysis and death) at high concentrations (> 0.1%) by causing leakage of all the major intracellular components out of the cell [26, 27].
Mouthwashes containing chlorhexidine are considered the gold standard [20]. Research findings have shown that chlorhexidine changes the nitrate-reducing bacteria in the body. Chlorhexidine seems to change the pH and lactate, nitrate, and nitrite concentrations in saliva, and chlorhexidine-containing mouthwashes may therefore lead to increased acidity and decreased nitrite concentration in the saliva by causing a major change in the salivary microbiome [28]. Chlorhexidine is a guanidine-based disinfectant that affects a wide variety of bacteria, fungi, and some viruses [29], but there is some controversy on its use [30]. Nevertheless, chlorhexidine has numerous side effects too [20], including parotid gland swelling, oral soft tissue pigmentation, allergic reactions, taste change, burning sensation, oral mucosa ulcers, transient anesthesia, and paresthesia. In addition, chlorhexidine can cause severe adverse effects in hypertensive patients by changing the oral microbiome and consequently reducing nitrate concentration in the saliva [31].
Traditional plant-based mouthwashes reduce unwanted reactions to chemicals such as antibiotic resistance and gradual decay or staining of teeth [18]. Herbal mouthwashes are produced from natural substances and have fewer side effects; they also have antibacterial, antiviral, and antifungal properties; therefore, their use has become widespread in the last decade [32]. The antibacterial effect of plant extracts and herbal mouthwashes against microorganisms responsible for bacterial infection of the oral cavity is attributed to the presence of compounds such as glycosides, terpenoids, flavonoids, and saponins [33].
Herbal mouthwashes containing cloves (Eugenia caryophyllata belonging to the Myrtaceae family) are used for oral cooling and in toothpastes and to treat oral diseases and toothache [34], or thousands of years, clove has been used as a traditional medicinal plant to treat gum infection due to its antimicrobial activities against oral bacteria [35, 36]. Eugenol, one of the main compounds of clove (81.1%), produces antibacterial, antifungal, anti-inflammatory, and antiviral effects and has been used as a disinfectant and pain reliever in traditional medicines [34, 37]. Clove has antimicrobial activities against oral bacteria and periodontal disease [36]. In the study by Mostaqim et al. (2019) in Bangladesh, the antibacterial efficacy of clove was demonstrated against gram-positive bacteria (Staphylococcus aureus) and gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa) [38]. The study by Purkait et al. (2020) in Germany showed the antibacterial and antifungal effects of combined clove and cinnamon on Staphylococcus aureus, Salmonella typhi, and Pseudomonas aeruginosa [39]. The study by Bharadwaj (2020) in India revealed the activity of the phytochemicals in clove extract and their antibacterial efficacy against Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella typhi, Bacillus subtilis, and Staphylococcus aureus [34]. Oral care is extensively used in the ICU for the prevention of VAP, and its effectiveness has been confirmed, but the exact details of its operational processes still require further discussion. So far, there is no gold standard recommendation for the scrubs used in oral care [40]. There is also no consensus on the best way of providing optimal oral health care in the critically ill. More research is required to standardize oral health assessment and care practices and thus develop evidence-based, individualized, oral care protocols for the critically ill [30, 41]. The British Association of Critical Nurses (BACCN) has also emphasized the need for further research on this topic [42]. The hypothesis of the present study was that clove mouthwash is effective in the incidence of VAP in MV patients. Therefore, the aim of the present study was to investigate the effect of clove mouthwash on the incidence of VAP in ICU patients.
Methods
Study design
This study was a comparative, randomized, triple-blind, clinical trial. The patients, the nurse who performed the mouthwash for them, the pulmonologist who made the diagnosis of VAP, and the statistician who carried out the data analysis were all blinded to the group allocations.
Inclusion criteria
The inclusion criteria consisted of age over 18 years, recent admission to the ICU and having undergone MV for more than 48 h, lack of aspiration symptoms or VAP with a Modified Clinical Pulmonary Infection Score (MCPIS) <6 on the first day, no COVID-19 infection, no face and mouth trauma, no contraindications for elevating the head of the bed by 30°, no contraindications for oral care, and no history of allergy to chlorhexidine 0.2%.
Exclusion criteria
The exclusion criteria were removal from MV at less than 48 h, fever of 38.5 °C and higher during the first 24 h after intubation, positive polymerase chain reaction (PCR) test (COVID-19 infection), more than three attempts at intubation, and APACHE II score > 30.
Participants
The study samples were the patients admitted to the internal and surgical ICUs of Kosar Hospital in Semnan in 2021–2022 who were connected to a mechanical ventilator and met the inclusion criteria.
Sample size
In a preliminary study consisting of 70 people in each group, 21.4% of patients receiving clove mouthwash and 41.4% of patients receiving chlorhexidine developed VAP (MCPIS score 6 or more). Considering 95% confidence and 80% power, the sample size was estimated to be 84 patients for each group.
Randomization
The patients who met the inclusion criteria were assigned to either intervention (A) or control (B) groups using random blocks. The possible states of allocation to either of the two blocks, i.e., AB or BA, were written on a card. Given that age and gender were confounding variables, after randomly choosing the first patient and assigning them to either of the blocks, the next patient, who could differ in age with the first patient by a maximum of 5 years, was assigned to the opposite group; otherwise, they waited for their turn in the next block. As for gender, stratified randomization was used—that is, randomization was done separately for men and women. In other words, each two female patients referring to the hospital consecutively were divided according to the above classifications in advance. The same was performed for male patients until sampling was completed.
Data collection instrument
Data were collected using a demographic questionnaire, VAP severity was measured based on the Acute Physiology and Chronic Health Evaluation II (APACHE II) score, oral hygiene status was determined using Beck Oral Assessment Scale (BOAS), and VAP diagnosis was made based on the Modified Clinical Pulmonary Infection Score (MCPIS).
Demographic questionnaire
The demographic questionnaire consisted of items on age, gender, occupation, underlying diseases; reason for ICU admission and ward from which ICU referral was made; ward where endotracheal intubation had been carried out; disease severity based on the APACHE II score; use of medications such as antibiotics, beta-blockers, antihistamines, diuretics, atropine, bronchodilators, immunosuppressants, and sedatives for dry mouth (xerostomia); history of smoking; score on the Glasgow Coma Scale; repeated attempts to perform intubation; accidental removal of the tracheal tube; MV parameters; the amount of PEEP used; the amount of pressure support; mode of MV device during hospitalization; spontaneous breathing and its duration; MV duration; ICU length of stay; and presence of nasogastric tube, gastric residual, and endotracheal tube cuff pressure.
Acute physiology and chronic health evaluation II (APACHE II)
The severity of the disease was measured using APACHE II criteria (Knaus et al., 1985) [43]. The APACHE II scoring system is a disease severity classification system and one of the most widely used scores in the ICU [44]. The score is calculated by measuring 12 physiological variables, age, and health status. The 12 physiological variables include body temperature, mean arterial pressure, heart rate, respiration rate, ratio of arterial oxygen partial pressure (Pao2) to fractional inspired oxygen (FiO2) (Pao2/FiO2 ratio), arterial pH, arterial HCO3, serum sodium level, serum potassium, hematocrit, creatinine, and leukocytes [44], and the total score ranges from 0 to 4. This scoring system works by focusing on the most unusual measurements and values in the first 24 h of ICU admission. The consciousness level was scored using the Glasgow Coma Scale. Scoring in the second (adjustment of age) and third (adjustment of underlying disease) parts was performed based on the specific classification from the APACHE II score form [45]. As for disease severity, APACHE II score < 16 was indicative of low, 16–25 moderate, 26–30 severe, and > 30 extremely severe disease [29]. This tool has acceptable validity and reliability [46].
Beck oral assessment scale (BOAS)
The oral health status of the patients was evaluated using BOAS scale. BOAS has been proposed as the most appropriate tool for use among ICU patients, with the mucosal-dental plaque score being the most applicable during observation [47], with five criteria, including the lips, gingival and oral mucosa membrane, tongue, teeth, and saliva. Each criterion was assigned a score from 1 to 4, so that the total score ranged from 5 to 20. The absence of disorder was scored as 5, mild disorder as 6–10, moderate disorder as 11–15, and severe disorder as 16–20 [29, 48]. This scoring system has satisfactory validity and reliability [49].
Modified clinical pulmonary infection score (MCPIS)
The diagnosis of VAP was made using the MCPIS by Fartukh et al. (2003) [50]. In this scoring system, the maximum score is 10, where MCPIS < 6 indicates the absence of VAP and MCPIS ≥ 6 indicates the presence of VAP. This system has been approved by the American Thoracic Society and the Infectious Diseases Society of America [11]. This scale evaluates five parameters, including body temperature, white blood cell count, secretions, the Pao2/FiO2 ratio, and chest X-ray. For each of these five parameters, based on the patient’s condition, a score of 0 to 2 is assigned, where 0 represents normal conditions and 1 and 2 represent lack of normal conditions. Body temperature was measured in the armpit. Temperatures of 36.5–38.4 °C received a score of 0, 38.5–39 °C a score of 1, and > 39 °C a score of 2. A white blood cell count of 4000–11,000 was given a score of 0, 11,000–17,000 score of 1, and over 17,000 score of 2. If the patient did not have secretions or was normal, they would be assigned a score of 0, and if they had secretions, they would be assigned a score of 1 or 2 depending on the amount of secretions and the physician’s opinion. The Pao2/FiO2 ratio was calculated by examining the arterial gasses and oxygen percentage set for MV. A ratio more than 200 was scored as 0, and a ratio less than 200 was scored as 2. The chest X-rays were checked by the physician. If the X-ray showed no problems, it was scored as 0, and X-rays containing scattered spots were scored as 1 and those with concentrated spots as 2. The scores of all the parameters were summed up, with the total score ranging from 0 to10. On the 1st and 5th days, the patients were examined for VAP by a pulmonologist using the MCPIS, and those with a score ≥ 6 were regarded as a case of VAP [11, 50]. This scale has demonstrated acceptable validity and reliability [11].
Protocol
Clove mouthwash solution at 6.66% concentration (Dr. Jahangir Pharmaceutical and Hygienic Co., Lorestan, Iran) and chlorhexidine 0.2% (Donyaye Behdasht Pharmaceutical Co., Tehran, Iran) were blindly provided to the ICU nurse by the researcher, and the nurse applied the mouthwash using 4–6 swabs.
In the intervention group, mouthwash was applied with 15 ml of clove extract at 6.66% concentration twice a day at 8:00 a.m. and 4:00 p.m. for 5 days.
In the control group, mouthwash was applied with 15 ml of chlorhexidine 0.2% (routine care) twice a day at 8:00 a.m. and 4:00 p.m. for 5 days.
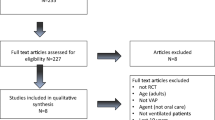
The first mouthwash was applied by a trained ICU nurse within the first 24 h of hospitalization and then on the second, third, fourth, and fifth days. The conditions of the procedure were the same in both groups. After washing the hands, to prevent the aspiration of secretions, the head of the patient’s bed was elevated by 30°, and the patient was positioned semi-sitting. Tracheal tube cuff pressure was measured using Covidien (Medtronic, Germany) and was maintained at 25 cm H2O. Using sterile gloves, the nurse soaked 4–6 swabs (depending on the patient’s oral hygiene status) with the solutions prepared for the intervention and control groups and washed the mucous membrane of their mouth, tongue and gums, and then suctioned the excess secretions. The suction conditions were the same for both groups [51]. Finally, the tape around the tracheal tube was replaced, the lips were cleaned, and some Vaseline was applied to lubricate the lip surface using an applicator. On the 1st and 5th days, the patients were examined for VAP using the MCPIS. A score of ≥ 6 was taken as having acquired VAP (Fig. 1).
Data analysis
The data obtained were analyzed using the Kolmogorov–Smirnov test, Mann–Whitney U-test, chi-square test, Fisher’s exact test, and the relative risk (RR) calculation at 95% CI in SPSS version 24, and the significance level (p value) was set at < 0.05.
Ethical considerations
An ethics code was obtained at Semnan University of Medical Sciences, and the study protocol was registered at the Iranian Registry of Clinical Trials. Necessary permissions were also obtained from the hospital and ICU authorities of Kosar Hospital. Before beginning the study procedure, the patient companions were briefed on the study objectives and procedure, and they then provided written informed consent for the patients’ participation in the study.
Results
Patients’ demographics
The mean ± standard deviation of age was 55.21 ± 9.7 and 59.7 ± 21.4 years in the intervention and control groups. The distribution of age (p = 0.267), gender (p = 0.419), occupation (p = 0.170), underlying disease (p > 0.05), medication use (p > 0.05), smoking (p = 0.278), disease severity (p = 0.412), oral hygiene status (p = 0.239), consciousness level (p = 0.819), reason for hospitalization (p = 0.236), ward from which ICU referral was made (p = 0.416), ward where intubation had been performed (p = 0.223), and pressure support (p = 0.736) did not differ significantly between the two groups (Table 1).
Outcome measures
APACHE II score was determined based on the physiological score (p = 0.414), age (p = 0.324), and underlying disease (p = 0.642), and there was no significant difference between the intervention and control groups in this regard (Table 2).
The baseline BOAS score (p = 0.239) for the lips (p = 0.018), gingival and oral mucous membrane (p = 0.116), tongue (p = 0.651), teeth (p = 0.074), and saliva (p = 0.673) showed no significant differences between the intervention and control groups (Table 3).
MCPIS for body temperature (p = 0.234), white blood cell count (p = 0.249), the PaO2/FiO2 ratio (p = 0.530), and chest X-ray (p = 0.097) showed no significant differences between the two groups; however, MCPIS (p = 0.002) and the secretions score (p = 0.008) differed significantly between the intervention and control groups (Table 4).
After the intervention, 20.2% (n = 17) of the patients in the intervention group and 41.7% (n = 35) in the control group acquired VAP. The incidence rate of VAP was 2.06 times higher in the control group than in the intervention group (RR = 2.06, 95% CI: 1.26–3.37, p = 0.005) (Table 5).
There was no significant difference in VAP severity between the two groups (p = 0.557) (Table 6).
Discussion
The results of this study showed that 20.2% of the patients in the intervention group and 41.7% of those in the control group acquired VAP. The risk of VAP was 2.06 times higher in the control group than in the intervention group. In line with the present findings, various other studies have also demonstrated the antibacterial and antimicrobial effects of clove. A study by Rajeshkumar et al. (2022) in India showed that chamomile and clove mouthwash has an optimal, dose-dependent, inhibitory role against Streptococcus mutans; therefore, chamomile and clove mouthwash could be a safe alternative for infections caused by S. mutans [52]. A study by Gupta et al. (2021) in India also revealed the antimicrobial activity of clove against the main bacterial causes of dental plaque, including Pseudomonas, Lactobacillus, and Streptococcus mutans; clove thus has the potential to be used as a natural antibacterial agent for oral pathogens [53]. A study by Ahmed et al. (2021) in Pakistan also showed that clove strongly affects the growth of different streptococcus species causing tooth decay [54]. A study by Sahu et al. (2020) in India showed that clove extract contains some compounds such as acetyleugenol, beta-caryophyllene, and vanillin which can be effective in the treatment of bronchitis [55]. A study by Green et al. (2020) further demonstrated the antiviral effect of clove extract by directly affecting the respiratory syncytial virus and eliminating its infectious properties [56]. A study by Nirmala et al. (2019) in India demonstrated the antimicrobial properties of clove against Staphylococcus aureus [37]. The study by Faujdar et al. (2020) in India revealed the antibacterial effects of clove extract on gram-negative bacteria [57]. Microscopic examinations carried out in a study by Wongsawan et al. (2020) in Thailand also showed the inability of Streptococcus to grow and its cell membrane disruption upon exposure to clove [58]. Furthermore, a study by Bharadwaj et al. (2020) in India showed the antimicrobial and phytochemical activity of clove against Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella typhi, Bacillus subtilis, and Staphylococcus aureus [34]. In addition, the study by Yassin et al. (2020) in Saudi Arabia revealed that clove is an effective, natural, and safe antifungal agent producing inhibitory effects against the most common and dominant types of candidiasis [59]. In contrast, a study by Mahajan (2016) in India found that the antimicrobial properties of tulsi (Ocimum tenuiflorum), clove, and neem (Azadirachta indica) mouthwash were lower compared to chlorhexidine [60]. In a study by Bansal et al. (2019) in India, the antimicrobial properties of clove were found to be lower than those of chlorhexidine [61]. Since the findings of the current study showed that clove mouthwash causes a significant reduction in VAP acquisition in ICU patients compared to chlorhexidine, due to the fewer side effects of herbal mouthwashes, the researchers recommend further studies as well as systematic reviews on this subject. This study showed differences in the secretions score of MCPIS between the two groups. Colonization, respiratory-digestive system and aspiration, contaminated secretions in the lower respiratory system, are two major processes responsible for VAP [62, 63].
The results of this study showed that the APACHE II scores did not differ significantly between the intervention and control groups. Other studies also found no statistically significant difference between the two groups regarding the APACHE-II score [11, 64]. The APACHE II score is a vital indicator of acute physiological abnormalities in patients [11, 64]. The APACHE II score is a vital indicator of acute physiological abnormalities in patients [65, 66].
Our research revealed that the majority of ICU patients had moderate oral health dysfunction. The results of a study by Malicka et al. (2020) in Poland showed moderate or severe dysfunction of oral health in older adults with poor oral health [67]. Also, hospitalization in an ICU along with MV can be harmful to oral health and lead to the buildup of dental plaque and emergence of mucosal lesions [68]. ICU patients are a group particularly susceptible to VAP because of the decline in their oral health [69]. In this study, BOAS did not differ significantly between the intervention and control groups. All ICU patients are at a high risk for developing VAP, but ensuring proper oral care in ICU patients has been shown to reduce the incidence of VAP [69, 70].
In our study, among the patients who acquired VAP, the severity of the disease did not differ significantly between the two groups. The study by Khaki et al. (2018) in Isfahan, Iran, conducted on the efficacy of Nanosil mouthwash for the prevention of pulmonary infection in ICU patients showed a significantly higher MCPIS in the control group, who had used chlorhexidine mouthwash, and the mean MCPIS was significantly higher in the control group on the fifth day than on the first day, but the mean MCPIS in the intervention group, who had used Nanosil mouthwash, did not show significant differences between the two intervals, i.e., at days 1 and 5 [71]. A comparative study by Babaiiet al. (2015) in Urmia, Iran, conducted on the effects of standard artificial airway care and routine care of the upper airway on the incidence of VAP showed that the mean MCPIS increased significantly on the third, fourth, and fifth days in the control group, who had received routine care without a specific protocol, with MCPIS increasing significantly on the fifth day compared to the third and fourth days. The mean MCPIS increased significantly on the third, fourth, and fifth days in the intervention group, but the increase was not statistically significant on the fifth day compared to the third and fourth days [72].
In the present study, bronchoalveolar lavage (BAL) was not performed due to its invasiveness, despite being a reliable and definitive method for the diagnosis of VAP. In addition, it was not possible to control several factors such as antibiotic resistance, differences between the patients’ immune systems, and resistant pathogens in the environment, which could have contributed to VAP incidence. Future comparative studies are recommended on the effects of clove mouthwash and other mouthwashes for the prevention of VAP, especially in ICU patients. Clove mouthwash was more effective than chlorhexidine for VAP incidence reduction. Nurses can use clove mouthwash in their care measures to reduce VAP acquisition.
Conclusion
The present study found that the use of clove extract mouthwash decreased the incidence of VAP in ICU patients undergoing MV compared to chlorhexidine. Therefore, clove mouthwash can be used as a simple and low-cost method to prevent VAP in ICU patients.
Data availability
The datasets generated and analyzed during the current study are not publicly available due to an agreement with the participants on the confidentiality of the data but are available from the corresponding author on reasonable request.
References
Fernando SM, Tran A, Cheng W, Klompas M, Kyeremanteng K, Mehta S, English SW, Muscedere J, Cook DJ, Torres A (2020) Diagnosis of ventilator-associated pneumonia in critically ill adult patients—a systematic review and meta-analysis. Intensive Care Med 46:1170–1179. https://doi.org/10.1007/s00134-020-06036-z
Zhao T, Wu X, Zhang Q, Li C, Worthington HV, Hua F (n.d.) Oral hygiene care for critically ill patients to prevent ventilator‐associated pneumonia. Cochrane Database of Syst Rev 2020: 1–139. https://doi.org/10.1002/14651858.CD008367
Gutiérrez JMM, Borromeo AR, Dueño AL, Paragas ED, Ellasus RO, Abalos-Fabia RS, Abriam JA, Sonido AE, Hernandez MA, Generale AJA (2019) Clinical epidemiology and outcomes of ventilator-associated pneumonia in critically ill adult patients: protocol for a large-scale systematic review and planned meta-analysis. Syst Rev 8(1):1–12. https://doi.org/10.1186/s13643-019-1080-y
Nusrat T, Akter N, Rahman NAA, Godman B, Rozario DTD, Haque M (2020) Antibiotic resistance and sensitivity pattern of metallo-β-lactamase producing gram-negative Bacilli in ventilator-associated pneumonia in the intensive care unit of a public medical school hospital in Bangladesh. Hosp Pract 48(3):128–36. https://doi.org/10.1080/21548331.2020.1754687
Farag AM, Tawfick MM, Abozeed MY, Shaban EA, Abo-Shadi MA (2020) Microbiological profile of ventilator-associated pneumonia among intensive care unit patients in tertiary Egyptian hospitals. J Infect Dev Countries 14(2):153–161. https://doi.org/10.3855/jidc.12012
Edwardson S, Cairns C (2019) Nosocomial infections in the ICU. Anaesth Intensive Care Med 20(1):14–18. https://doi.org/10.1016/j.mpaic.2018.11.004
Papazian L, Klompas M, Luyt C-E (2020) Ventilator-associated pneumonia in adults: a narrative review. Intensive Care Med 46(3):886–906. https://doi.org/10.1007/s00134-020-05980-0
Ding C, Zhang Y, Yang Z, Wang J, Jin A, Wang W, Chen R, Zhan S (2017) Incidence, temporal trend and factors associated with ventilator-associated pneumonia in mainland China: a systematic review and meta-analysis. BMC Infect Dis 17(1):1–10. https://doi.org/10.1186/s12879-017-2566-7
Li Y, Liu C, Xiao W, Song T, Wang S (2020) Incidence, risk factors, and outcomes of ventilator-associated pneumonia in traumatic brain injury: a meta-analysis. Neurocrit Care 32(1):272–285. https://doi.org/10.1007/s12028-019-00773-w
Bonell A, Azarrafiy R, Huong VTL, Viet TL, Phu VD, Dat VQ, Wertheim H, van Doorn HR, Lewycka S, Nadjm B (2019) A systematic review and meta-analysis of ventilator-associated pneumonia in adults in Asia: an analysis of national income level on incidence and etiology. Clin Infect Dis 68(3):511–518. https://doi.org/10.1093/cid/ciy543
Nobahar M, Razavi MR, Malek F, Ghorbani R (2016) Effects of hydrogen peroxide mouthwash on preventing ventilator-associated pneumonia in patients admitted to the intensive care unit. Braz J Infect Dis 20(5):444–450. https://doi.org/10.1016/j.bjid.2016.06.005
Wu D, Wu C, Zhang S, Zhong Y (2019) Risk factors of ventilator-associated pneumonia in critically III patients. Front Pharmacol 10:482. https://doi.org/10.3389/fphar.2019.00482
Yekefallah L, Shafaei M, Dehghankar L (2019) Strategies for the prevention of ventilator-associated pneumonia in the intensive care units: a review. J Qazvin Univ Med Sci 23(4):352–363
Jones DJ, Munro CL, Grap MJ (2011) Natural history of dental plaque accumulation in mechanically ventilated adults: a descriptive correlational study. Intensive Crit Care Nurs 27(6):299–304. https://doi.org/10.1016/j.iccn.2011.08.005
Needleman I, Hyun-Ryu J, Brealey D, Sachdev M, Moskal-Fitzpatrick D, Bercades G, Nagle J, Lewis K, Agudo E, Petrie A (2012) The impact of hospitalization on dental plaque accumulation: an observational study. J Clin Periodontol 39(11):1011–1016. https://doi.org/10.1111/j.1600-051X.2012.01939.x
Landgraf ACM, Reinheimer A, Merlin JC, Couto SdAB, Souza PHC (2017) Mechanical ventilation and cytopathological changes in the oral mucosa. Am J Crit Care 26(4):297–302. https://doi.org/10.4037/ajcc2017218
Takahama A Jr, de Sousa VI, Tanaka EE, Ono E, Ito FAN, Costa PP, Pedriali MBBP, de Lima HG, Fornazieri MA, Correia LS (2021) Analysis of oral risk factors for ventilator-associated pneumonia in critically ill patients. Clin Oral Invest 25(3):1217–1222. https://doi.org/10.1007/s00784-020-03426-x
Harput US (2020) Herbal products for oral hygiene: an overview of their biological activities. Nat Oral Care Dent Ther 30:31–44. https://doi.org/10.1002/9781119618973.ch2
Ory J, Mourgues C, Raybaud E, Chabanne R, Jourdy JC, Belard F, Guérin R, Cosserant B, Faure JS, Calvet L (2018) Cost assessment of a new oral care program in the intensive care unit to prevent ventilator-associated pneumonia. Clin Oral Invest 22(5):1945–1951. https://doi.org/10.1007/s00784-017-2289-6
Carolina A, Ferreira J (2021) Efficiency of different protocols for oral hygiene combined with the use of chlorhexidine in the prevention of ventilator-associated pneumonia. J Bras Pneumo 47(1):1–8. https://doi.org/10.36416/1806-3756/e20190286
Silva PUJ, Paranhos LR, Meneses-Santos D, Blumenberg C, Macedo DR, Cardoso SV (2021) Combination of toothbrushing and chlorhexidine compared with exclusive use of chlorhexidine to reduce the risk of ventilator-associated pneumonia: a systematic review with meta-analysis. Clinics 76:e2659. https://doi.org/10.6061/clinics/2021/e2659
Septimus EJ, Schweizer ML (2016) Decolonization in prevention of health care-associated infections. Clin Microbiol Rev 29(2):201–222. https://doi.org/10.1128/CMR.00049-15
Kumar SB (2017) Chlorhexidine mouthwash-a review. J Pharm Sci Res 9(9):1450
Jackson L, Owens M (2019) Does oral care with chlorhexidine reduce ventilator-associated pneumonia in mechanically ventilated adults? Br J Nurs 28(11):682–9. https://doi.org/10.12968/bjon.2019.28.11.682
Chacko R, Rajan A, Lionel P, Thilagavathi M, Yadav B, Premkumar J (2017) Oral decontamination techniques and ventilator-associated pneumonia. Br J Nurs 26(11):594–9. https://doi.org/10.12968/bjon.2017.26.11.594
Brookes ZL, Bescos R, Belfield LA, Ali K, Roberts A (2020) Current uses of chlorhexidine for management of oral disease: a narrative review. J Dent 103:103497. https://doi.org/10.1016/j.jdent.2020.103497
Cieplik F, Jakubovics NS, Buchalla W, Maisch T, Hellwig E, Al-Ahmad A (2019) Resistance toward chlorhexidine in oral bacteria–is there cause for concern? Front Microbiol 10:587. https://doi.org/10.3389/fmicb.2019.00587
Brookes ZL, Belfield LA, Ashworth A, Casas-Agustench P, Raja M, Pollard AJ, Bescos R (2021) Effects of chlorhexidine mouthwash on the oral microbiome. J Dent 113:103768. https://doi.org/10.1016/j.jdent.2021.103768
Anggraeni DT, Hayati AT, Nur’aeni A (2020) The effect of oral care intervention on oral health status of intubated patiant in the intensive care unit. Belitung Nurs J 6(1):21–6. https://doi.org/10.33546/bnj.971
Winning L, Lundy FT, Blackwood B, McAuley DF, El Karim I (2021) Oral health care for the critically ill: a narrative review. Crit Care 25(1):1–8. https://doi.org/10.1186/s13054-021-03765-5
Pałka Ł, Nowakowska-Toporowska A, Dalewski B, editors (n.d.) Is chlorhexidine in dentistry an ally or a foe? A narrative review. Healthcare; 2022: MDPI. https://doi.org/10.3390/healthcare10050764
Batiha GE-S, Alkazmi LM, Wasef LG, Beshbishy AM, Nadwa EH, Rashwan EK (2020) Syzygium aromaticum L. (Myrtaceae): traditional uses, bioactive chemical constituents, pharmacological and toxicological activities. Biomolecules 10(2):202. https://doi.org/10.3390/biom10030352
Yadav AR, Mohite SK, Magdum CS (2020) Preparation and evaluation of antibacterial herbal mouthwash against oral pathogens. Asian J Res Pharm Sci 10(3). https://doi.org/10.5958/2231-5659.2020.00028.4
Bharadwaj A (n.d.) Qualitative and quantitative analysis of phytochemicals and antimicrobial activity of Syzygium aromaticum. Available at SSRN 3554164 2020: 1-14. https://doi.org/10.2139/ssrn.3554164
Hepokur C (2018) Investigation of cytotoxic effects of Eugenia caryophyllus (Clove). Cumhuriyet Dent J 21(3):173–177. https://doi.org/10.7126/cumudj.444426
Liu Q, Meng X, Li Y, Zhao C-N, Tang G-Y, Li H-B (2017) Antibacterial and antifungal activities of spices. Int J Mol Sci 18(6):1283. https://doi.org/10.3390/ijms18061283
Nirmala MJ, Durai L, Gopakumar V, Nagarajan R (2019) Anticancer and antibacterial effects of a clove bud essential oil-based nanoscale emulsion system. Int J Nanomed 14:6439. https://doi.org/10.2147/IJN.S211047
Mostaqim S, Saha S, Hani U, Paul S, Sharmin M, Basak S, Begum S, Salma U, Shahabuddin M (2019) Antibacterial activities of clove (Syzygium aromaticum) extracts against three food borne pathogens: Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. Mymensingh Med J: MMJ 28(4):779–791
Purkait S, Bhattacharya A, Bag A, Chattopadhyay R (2020) Synergistic antibacterial, antifungal and antioxidant efficacy of cinnamon and clove essential oils in combination. Arch Microbiol 202:1439–1448. https://doi.org/10.1007/s00203-020-01858-3
Wei H-p, Yang K (2019) Effects of different oral care scrubs on ventilator-associated pneumonia prevention for machinery ventilates patient: a protocol for systematic review, evidence mapping, and network meta-analysis. Medicine 98(12):e14923. https://doi.org/10.1097/MD.0000000000014923
Dale CM, Rose L, Carbone S, Pinto R, Smith OM, Burry L, Fan E, Amaral ACK-B, McCredie VA, Scales DC (2021) Effect of oral chlorhexidine de-adoption and implementation of an oral care bundle on mortality for mechanically ventilated patients in the intensive care unit (CHORAL): a multi-center stepped wedge cluster-randomized controlled trial. Intensive Care Med 47(11):1295–302. https://doi.org/10.1007/s00134-021-06475-2
Collins T, Plowright C, Gibson V, Stayt L, Clarke S, Caisley J, Watkins CH, Hodges E, Leaver G, Leyland S (2021) British Association of Critical Care Nurses: evidence-based consensus paper for oral care within adult critical care units. Nurs Crit Care 26(4):224–233. https://doi.org/10.1111/nicc.12570
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13(10):818–829
Sutiono AB, Arifin MZ, Adhipratama H, Hermanto Y (2022) The utilization of APACHE II score to predict the incidence of ventilator-associated pneumonia in patients with severe traumatic brain injury: a single-center study. Interdiscip Neurosurg 28:101457. https://doi.org/10.1016/j.inat.2021.101457
TavakoliGhuchani H, Sadat Hejazi S, Ebrahimi B, Tabei M (2018) Mortality rate of patients admitted to the intensive care unit of Shahid Chamran Hospital of Ferdows City with Acute Physiology and Chronic Health Evaluation (APACHE) II. J N Khorasan Univ Med Sci 10(1):30–35
Czajka S, Ziębińska K, Marczenko K, Posmyk B, Szczepańska AJ, Krzych ŁJ (2020) Validation of APACHE II, APACHE III and SAPS II scores in in-hospital and one year mortality prediction in a mixed intensive care unit in Poland: a cohort study. BMC Anesthesiol 20(1):1–8. https://doi.org/10.1186/s12871-020-01203-7
Ames NJ, Sulima P, Yates JM, McCullagh L, Gollins SL, Soeken K, Wallen GR (2011) Effects of systematic oral care in critically ill patients: a multicenter study. Am J Crit Care 20(5):e103–e114. https://doi.org/10.4037/ajcc2011359
Gupta A, Gupta A, Singh T, Saxsena A (2016) Role of oral care to prevent VAP in mechanically ventilated intensive care unit patients. Saudi J Anaesth 10(1):95. https://doi.org/10.4103/1658-354X.169484
Handa S, Chand S, Sarin J, Singh VA, Sharma S (2014) Effectiveness of oral care protocal on oral health status of hospitalised children admitted in Intensive Care Units of selected hospital of Haryana. Nurs Midwifery Res J 10(1):8–15. https://doi.org/10.1177/0974150X20140102
Fartoukh M, Maître B, Honoré S, Cerf C, Zahar J-R, Brun-Buisson C (2003) Diagnosing pneumonia during mechanical ventilation: the clinical pulmonary infection score revisited. Am J Respir Crit Care Med 168(2):173–179. https://doi.org/10.1164/rccm.200212-1449OC
Pozuelo-Carrascosa DP, Herráiz-Adillo Á, Alvarez-Bueno C, Añón JM, Martínez-Vizcaíno V, Cavero-Redondo I (2020) Subglottic secretion drainage for preventing ventilator-associated pneumonia: an overview of systematic reviews and an updated meta-analysis. Eur Respir Rev 29(151):190107. https://doi.org/10.1183/16000617.0107-2019
Amrithaa B, Cecil A, Rajeshkumar S (2022) Comparative antimicrobial activity of chamomile and clove based mouthwash with commercial mouthwash. Int J Early Child Spec Educ (INT-JECSE) 14:5445–5450. https://doi.org/10.9756/INT-JECSE/V14I2.609
Gupta C, Prakash D (2021) Comparative study of the antimicrobial activity of clove oil and clove extract on oral pathogens. Dent - Open J 6(1):12–15. https://doi.org/10.1016/j.carbpol.2020.117339
Ahmed NM, Tariq P, Naim A (2021) Antimicrobial activities of Clove Buds and Miswak againts Viridans group Streptococci. INT J BIOL BIOTECH 18(2):247–252
Sahu S, Sahoo AK, Swain S, Bhattacharyay D (2020) In silico analysis of phytochemicals from clove against bronchitis. Eur J Med Plants 20:34–39. https://doi.org/10.9734/EJMP/2020/v31i530239
Green A (n.d.) Anti-respiratory syncytial virus activity of clove extract,University of Gothenburg / Institute of Medicine. Sahlgrenska AcademyInstitute of biomedicine 2020: 1–31
Faujdar SS, Bisht D, Sharma A (2020) Antibacterial activity of Syzygium aromaticum (clove) against uropathogens producing ESBL, MBL, and AmpC beta-lactamase: are we close to getting a new antibacterial agent? J Fam Med Prim Care 9(1):180. https://doi.org/10.4103/jfmpc.jfmpc_908_19
Wongsawan K, Chaisri W, Tangtrongsup S, Mektrirat R (2020) Bactericidal effect of clove oil against multidrug-resistant Streptococcus suis isolated from human patients and slaughtered pigs. Pathogens 9(1):14. https://doi.org/10.3390/pathogens9010014
Yassin MT, Mostafa AA-F, Al-Askar AA (2020) In vitro anticandidal potency of Syzygium aromaticum (clove) extracts against vaginal candidiasis. BMC Complement Med Ther 20(1):25. https://doi.org/10.1186/s12906-020-2818-8
Mahajan R, Khinda PK, Gill AS, Kaur J, Saravanan S, Sahewal A, Taneja M, Joshi V (2016) Comparison of efficacy of 0.2% chlorhexidine gluconate and herbal mouthrinses on dental plaque: an in vitro comparative study. Eur J Med Plants 13(2):1. https://doi.org/10.9734/EJMP/2016/23318
Bansal V, Gupta M, Bhaduri T, Shaikh SA, Sayed FR, Bansal V, Agrawal A (2019) Assessment of antimicrobial effectiveness of neem and clove extract against Streptococcus mutans and Candida albicans: an in vitro study. Niger Med J: J Niger Med Assoc 60(6):285. https://doi.org/10.4103/nmj.NMJ_20_19
Pacheco-Fowler V, Gaonkar T, Wyer P, Modak S (2004) Antiseptic impregnated endotracheal tubes for the prevention of bacterial colonization. J Hosp Infect 57(2):170–174. https://doi.org/10.1016/j.jhin.2004.03.011
van Nieuwenhoven CA, Vandenbroucke-Grauls C, van Tiel FH, Joore HC, van Schijndel RJS, van der Tweel I, Ramsay G, Bonten MJ (2006) Feasibility and effects of the semirecumbent position to prevent ventilator-associated pneumonia: a randomized study. Crit Care Med 34(2):396–402. https://doi.org/10.1097/01.ccm.0000198529.76602.5e
Younes SAR, Ahmed NT, Ahmed IM, Hassan EA (2022) Effect of multimodality chest physiotherapy interventions on prevention of ventilator associated pneumonia among mechanically ventilated patients. Alex Sci Nurs J 24(1):36–46
Erinc A, Kutbay Ozcelik H, Yigitbas BA, Yurt S, Kosar F (2018) Does subglottic secretion drainage prevent ventilator-associated pneumonia? Eurasian J Pulmonol 20(3):122. https://doi.org/10.4103/ejop.ejop
Luyt C-E, Hékimian G, Koulenti D, Chastre J (2018) Microbial cause of ICU-acquired pneumonia: hospital-acquired pneumonia versus ventilator-associated pneumonia. Curr Opin Crit Care 24(5):332–338. https://doi.org/10.1097/MCC.0000000000000526
Malicka B, Skośkiewicz-Malinowska K, Kaczmarek U (2022) The impact of socioeconomic status, general health and oral health on Health-Related Quality of Life, Oral Health-Related Quality of Life and mental health among Polish older adults. BMC Geriatr 22(1):1–15. https://doi.org/10.1186/s12877-021-02716-7
Terezakis E, Needleman I, Kumar N, Moles D, Agudo E (2011) The impact of hospitalization on oral health: a systematic review. J Clin Periodontol 38(7):628–636. https://doi.org/10.1111/j.1600-051X.2011.01727.x
Luyt C-E, Sahnoun T, Gautier M, Vidal P, Burrel S, Pineton de Chambrun M, Chommeloux J, Desnos C, Arzoine J, Nieszkowska A (2020) Ventilator-associated pneumonia in patients with SARS-CoV-2-associated acute respiratory distress syndrome requiring ECMO: a retrospective cohort study. Ann Intensive Care 10(1):1–10. https://doi.org/10.1186/s13613-020-00775-4
Bao L, Zhang C, Dong J, Zhao L, Li Y, Sun J (2020) Oral microbiome and SARS-CoV-2: beware of lung co-infection. Front Microbiol 11:1840. https://doi.org/10.3389/fmicb.2020.01840
Khaky B, Yazdannik A, Mahjobipoor H (2018) Evaluating the efficacy of nanosil mouthwash on the preventing pulmonary infection in intensive care unit: a randomized clinical trial. Med Arch 72(3):206. https://doi.org/10.5455/medarh.2018.72.206-209
Babaii A, Abbasinia M, Bahrami N (2016) The effect of artificial airway standardization cares on the ventilator-associated pneumonia. J Urmia Nurs Midwifery Fac 13(11):987–994
Author information
Authors and Affiliations
Contributions
All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Mojgan Jahanshir: conceptualization, methodology, investigation, data curation, writing—original draft
Monir Nobahar: conceptualization, methodology, investigation, data curation; writing—original draft, writing—review and editing, supervision, project administration
Raheb Ghorbani: conceptualization, data curation; writing—original draft, writing—review and editing
Farhad Malek: conceptualization, data curation; writing—original draft, writing—review and editing
Corresponding author
Ethics declarations
Ethical approval
We adhered to the ethical considerations by obtaining a license from the Semnan Ethics Committee (IR.SEMUMS.REC.1399.184), on 22.09.2020, the Iran Clinical Trial Registration Center IRCT20110427006318N13, and the vice-chancellor for research at Semnan University of Medical Sciences, explaining the research objectives to the participants and obtaining their informed consent to participate in the research, ensuring them of the voluntary participation in the study and the confidentiality terms. In addition, we appreciated the cooperation of the subjects and the authorities for their cooperation with the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jahanshir, M., Nobahar, M., Ghorbani, R. et al. Effect of clove mouthwash on the incidence of ventilator-associated pneumonia in intensive care unit patients: a comparative randomized triple-blind clinical trial. Clin Oral Invest 27, 3589–3600 (2023). https://doi.org/10.1007/s00784-023-04972-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-023-04972-w