Abstract
Objective
To compare the in vitro decontamination efficacy of two electrolytic cleaning methods to diode laser, plasma, and air-abrasive devices.
Material and methods
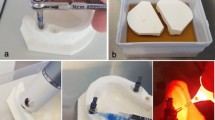
Sixty sandblasted large-grit acid-etched (SLA) implants were incubated with 2 ml of human saliva and Tryptic Soy Broth solution under continuous shaking for 14 days. Implants were then randomly assigned to one untreated control group (n = 10) and 5 different decontamination modalities: air-abrasive powder (n = 10), diode laser (n = 10), plasma cleaning (n = 10), and two electrolytic test protocols using either potassium iodide (KI) (n = 10) or sodium formate (CHNaO2) (n = 10) solution. Implants were stained for dead and alive bacteria in two standardized measurement areas, observed at fluorescent microscope, and analyzed for color intensity.
Results
All disinfecting treatment modalities significantly reduced the stained area compared to the untreated control group for both measurement areas (p < 0.001). Among test interventions, electrolytic KI and CHNaO2 treatments were equally effective, and each one significantly reduced the stained area compared to any other treatment modality (p < 0.001). Efficacy of electrolytic protocols was not affected by the angulation of examined surfaces [surface angulation 0° vs. 60° (staining %): electrolytic cleaning-KI 0.03 ± 0.04 vs. 0.09 ± 0.10; electrolytic cleaning-CHNaO2 0.01 ± 0.01 vs. 0.06 ± 0.08; (p > 0.05)], while air abrasion [surface angulation 0° vs. 60° (staining %): 2.66 ± 0.83 vs. 42.12 ± 3.46 (p < 0.001)] and plasma cleaning [surface angulation 0° vs. 60° (staining %): 33.25 ± 3.01 vs. 39.16 ± 3.15 (p < 0.001)] were.
Conclusions
Within the limitations of the present in vitro study, electrolytic decontamination with KI and CHNaO2 was significantly more effective in reducing bacterial stained surface of rough titanium implants than air-abrasive powder, diode laser, and plasma cleaning, regardless of the accessibility of the contaminated implant location.
Clinical relevance
Complete bacterial elimination (residual bacteria < 1%) was achieved only for the electrolytic cleaning approaches, irrespectively of the favorable or unfavorable access to implant surface.




Similar content being viewed by others
Data availability
The data sets used and analyzed during the current study are available as electronic supplementary material.
References
Berglundh T, Armitage G, Araujo MG, Avila-Ortiz G, Blanco J, Camargo PM, Chen S, Cochran D, Derks J, Figuero E, Hammerle CHF, Heitz-Mayfield LJA, Huynh-Ba G, Iacono V, Koo KT, Lambert F, McCauley L, Quirynen M, Renvert S, Salvi GE, Schwarz F, Tarnow D, Tomasi C, Wang HL, Zitzmann N (2018) Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol 45(Suppl 20):S286–S291. https://doi.org/10.1111/jcpe.12957
Fu JH, Wang HL (2020) Breaking the wave of peri-implantitis. Periodontol 2000 84(1):145–160. https://doi.org/10.1111/prd.12335
Salvi GE, Aglietta M, Eick S, Sculean A, Lang NP, Ramseier CA (2012) Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clin Oral Implants Res 23(2):182–190. https://doi.org/10.1111/j.1600-0501.2011.02220.x
Costa FO, Takenaka-Martinez S, Cota LO, Ferreira SD, Silva GL, Costa JE (2012) Peri-implant disease in subjects with and without preventive maintenance: a 5-year follow-up. J Clin Periodontol 39(2):173–181. https://doi.org/10.1111/j.1600-051X.2011.01819.x
Canullo L, Penarrocha M, Monje A, Catena A, Wang HL, Penarrocha D (2017) Association between clinical and microbiologic cluster profiles and peri-implantitis. Int J Oral Maxillofac Implants 32(5):1054–1064. https://doi.org/10.11607/jomi.6043
Canullo L, Schlee M, Wagner W, Covani U, Montegrotto Group for the study of peri-implant D (2015) International Brainstorming Meeting on Etiologic and Risk Factors of Peri-implantitis, Montegrotto (Padua, Italy), August 2014. Int J Oral Maxillofac Implants 30(5):1093–1104. https://doi.org/10.11607/jomi.4386
Derks J, Hakansson J, Wennstrom JL, Tomasi C, Larsson M, Berglundh T (2015) Effectiveness of implant therapy analyzed in a Swedish population: early and late implant loss. J Dent Res 94(3 Suppl):44S-51S. https://doi.org/10.1177/0022034514563077
Wilson TG Jr (2021) Bone loss around implants-is it metallosis? J Periodontol 92(2):181–185. https://doi.org/10.1002/JPER.20-0208
Karlsson K, Derks J, Wennstrom JL, Petzold M, Berglundh T (2020) Occurrence and clustering of complications in implant dentistry. Clin Oral Implants Res 31(10):1002–1009. https://doi.org/10.1111/clr.13647
Heitz-Mayfield LJA, Heitz F, Lang NP (2020) Implant Disease Risk Assessment IDRA-a tool for preventing peri-implant disease. Clin Oral Implants Res 31(4):397–403. https://doi.org/10.1111/clr.13585
Wang CW, Hao Y, Di Gianfilippo R, Sugai J, Li J, Gong W, Kornman KS, Wang HL, Kamada N, Xie Y, Giannobile WV, Lei YL (2021) Machine learning-assisted immune profiling stratifies peri-implantitis patients with unique microbial colonization and clinical outcomes. Theranostics 11(14):6703–6716. https://doi.org/10.7150/thno.57775
Jakubovics NS, Goodman SD, Mashburn-Warren L, Stafford GP, Cieplik F (2021) The dental plaque biofilm matrix. Periodontol 2000 86(1):32–56. https://doi.org/10.1111/prd.12361
Joseph S, Curtis MA (2021) Microbial transitions from health to disease. Periodontol 2000 86(1):201–209. https://doi.org/10.1111/prd.12377
Darveau RP, Curtis MA (2021) Oral biofilms revisited: a novel host tissue of bacteriological origin. Periodontol 2000 86(1):8–13. https://doi.org/10.1111/prd.12374
Bermejo P, Sanchez MC, Llama-Palacios A, Figuero E, Herrera D, Sanz M (2019) Topographic characterization of multispecies biofilms growing on dental implant surfaces: an in vitro model. Clin Oral Implants Res 30(3):229–241. https://doi.org/10.1111/clr.13409
Sirinirund B, Garaicoa-Pazmino C, Wang HL (2019) Effects of mechanical instrumentation with commercially available instruments used in supportive peri-implant therapy: an in vitro study. Int J Oral Maxillofac Implants 34(6):1370–1378. https://doi.org/10.11607/jomi.7409
Sahrmann P, Ronay V, Hofer D, Attin T, Jung RE, Schmidlin PR (2015) In vitro cleaning potential of three different implant debridement methods. Clin Oral Implants Res 26(3):314–319. https://doi.org/10.1111/clr.12322
Heitz-Mayfield LJ, Mombelli A (2014) The therapy of peri-implantitis: a systematic review. Int J Oral Maxillofac Implants 29(Suppl):325–345. https://doi.org/10.11607/jomi.2014suppl.g5.3
Di Gianfilippo R, Sirinirund B, Rodriguez VM, Chen Z, Wang H-L (2020) Long-term prognosis of peri-implantitis treatment: a systematic review of prospective trials with more than 3 years of follow-up. Appl Sci 10(24):9084. https://doi.org/10.3390/app10249084
Kaan AMM, Kahharova D (2000) Zaura E (2021) Acquisition and establishment of the oral microbiota. Periodontol 86(1):123–141. https://doi.org/10.1111/prd.12366
Kotsakis GA, Olmedo DG (2021) Peri-implantitis is not periodontitis: scientific discoveries shed light on microbiome-biomaterial interactions that may determine disease phenotype. Periodontol 2000 86(1):231–240. https://doi.org/10.1111/prd.12372
Franceschi D, Giuliani V, Giuntini V, Pini Prato GP, Chambrone L (2021) Retrograde peri-implantitis: report of a case successfully treated by resection of the implant apex. Int J Periodontics Restorative Dent 41(3):443–448. https://doi.org/10.11607/prd.4837
Di Gianfilippo R, Askar H, Henderson J, Franceschi D, Wang HL, Wang CW (2021) Intra- and Inter-examiner repeatability of diagnostic peri-implant clinical measurement: a pilot study. J Oral Implantol. https://doi.org/10.1563/aaid-joi-D-20-00160
Cha JK, Paeng K, Jung UW, Choi SH, Sanz M, Sanz-Martin I (2019) The effect of five mechanical instrumentation protocols on implant surface topography and roughness: a scanning electron microscope and confocal laser scanning microscope analysis. Clin Oral Implants Res 30(6):578–587. https://doi.org/10.1111/clr.13446
Mizutani K, Aoki A, Coluzzi D, Yukna R, Wang CY, Pavlic V, Izumi Y (2016) Lasers in minimally invasive periodontal and peri-implant therapy. Periodontol 2000 71(1):185–212. https://doi.org/10.1111/prd.12123
Wilson M, Burns T, Pratten J, Pearson GJ (1995) Bacteria in supragingival plaque samples can be killed by low-power laser light in the presence of a photosensitizer. J Appl Bacteriol 78(5):569–574. https://doi.org/10.1111/j.1365-2672.1995.tb03101.x
Dortbudak O, Haas R, Bernhart T, Mailath-Pokorny G (2001) Lethal photosensitization for decontamination of implant surfaces in the treatment of peri-implantitis. Clin Oral Implants Res 12(2):104–108. https://doi.org/10.1034/j.1600-0501.2001.012002104.x
Bassetti M, Schar D, Wicki B, Eick S, Ramseier CA, Arweiler NB, Sculean A, Salvi GE (2014) Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: 12-month outcomes of a randomized controlled clinical trial. Clin Oral Implants Res 25(3):279–287. https://doi.org/10.1111/clr.12155
Schar D, Ramseier CA, Eick S, Arweiler NB, Sculean A, Salvi GE (2013) Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: six-month outcomes of a prospective randomized clinical trial. Clin Oral Implants Res 24(1):104–110. https://doi.org/10.1111/j.1600-0501.2012.02494.x
Duske K, Koban I, Kindel E, Schroder K, Nebe B, Holtfreter B, Jablonowski L, Weltmann KD, Kocher T (2012) Atmospheric plasma enhances wettability and cell spreading on dental implant metals. J Clin Periodontol 39(4):400–407. https://doi.org/10.1111/j.1600-051X.2012.01853.x
Rupf S, Idlibi AN, Marrawi FA, Hannig M, Schubert A, von Mueller L, Spitzer W, Holtmann H, Lehmann A, Rueppell A, Schindler A (2011) Removing biofilms from microstructured titanium ex vivo: a novel approach using atmospheric plasma technology. PLoS ONE 6(10):e25893. https://doi.org/10.1371/journal.pone.0025893
Matthes R, Duske K, Kebede TG, Pink C, Schluter R, von Woedtke T, Weltmann KD, Kocher T, Jablonowski L (2017) Osteoblast growth, after cleaning of biofilm-covered titanium discs with air-polishing and cold plasma. J Clin Periodontol 44(6):672–680. https://doi.org/10.1111/jcpe.12720
Schlee M, Wang HL, Stumpf T, Brodbeck U, Bosshardt D, Rathe F (2021) Treatment of periimplantitis with electrolytic cleaning versus mechanical and electrolytic cleaning: 18-month results from a randomized controlled clinical Trial. J Clin Med 10(16):3475. https://doi.org/10.3390/jcm10163475
Ratka C, Weigl P, Henrich D, Koch F, Schlee M, Zipprich H (2019) The effect of in vitro electrolytic cleaning on biofilm-contaminated implant surfaces. J Clin Med 8(9):1397. https://doi.org/10.3390/jcm8091397
Schlee M, Rathe F, Brodbeck U, Ratka C, Weigl P, Zipprich H (2019) Treatment of peri-implantitis-electrolytic cleaning versus mechanical and electrolytic cleaning-a randomized controlled clinical trial-six-month results. J Clin Med 8(11):1909. https://doi.org/10.3390/jcm8111909
Schlee M, Naili L, Rathe F, Brodbeck U, Zipprich H (2020) Is complete re-osseointegration of an infected dental implant possible? histologic results of a dog study: a short communication. J Clin Med 9(1):235. https://doi.org/10.3390/jcm9010235
Idlibi AN, Al-Marrawi F, Hannig M, Lehmann A, Rueppell A, Schindler A, Jentsch H, Rupf S (2013) Destruction of oral biofilms formed in situ on machined titanium (Ti) surfaces by cold atmospheric plasma. Biofouling 29(4):369–379. https://doi.org/10.1080/08927014.2013.775255
Berglundh T, Jepsen S, Stadlinger B, Terheyden H (2019) Peri-implantitis and its prevention. Clin Oral Implants Res 30(2):150–155. https://doi.org/10.1111/clr.13401
Keim D, Nickles K, Dannewitz B, Ratka C, Eickholz P, Petsos H (2019) In vitro efficacy of three different implant surface decontamination methods in three different defect configurations. Clin Oral Implants Res 30(6):550–558. https://doi.org/10.1111/clr.13441
John G, Sahm N, Becker J, Schwarz F (2015) Nonsurgical treatment of peri-implantitis using an air-abrasive device or mechanical debridement and local application of chlorhexidine. Twelve-month follow-up of a prospective, randomized, controlled clinical study. Clin Oral Investig 19(8):1807–1814. https://doi.org/10.1007/s00784-015-1406-7
Ji YJ, Tang ZH, Wang R, Cao J, Cao CF, Jin LJ (2014) Effect of glycine powder air-polishing as an adjunct in the treatment of peri-implant mucositis: a pilot clinical trial. Clin Oral Implants Res 25(6):683–689. https://doi.org/10.1111/clr.12123
Renvert S, Lindahl C, Roos Jansaker AM, Persson GR (2011) Treatment of peri-implantitis using an Er:YAG laser or an air-abrasive device: a randomized clinical trial. J Clin Periodontol 38(1):65–73. https://doi.org/10.1111/j.1600-051X.2010.01646.x
Wang CW, Ashnagar S, Di Gianfilippo R, Arnett M, Kinney J, Wang HL (2021) Laser-assisted regenerative surgical therapy for peri-implantitis: a randomized controlled clinical trial. J Periodontol 92(3):378–388. https://doi.org/10.1002/JPER.20-0040
Kreisler M, Kohnen W, Marinello C, Gotz H, Duschner H, Jansen B, d’Hoedt B (2002) Bactericidal effect of the Er:YAG laser on dental implant surfaces: an in vitro study. J Periodontol 73(11):1292–1298. https://doi.org/10.1902/jop.2002.73.11.1292
Yan S, Li M, Komasa S, Agariguchi A, Yang Y, Zeng Y, Takao S, Zhang H, Tashiro Y, Kusumoto T, Kobayashi Y, Chen L, Kashiwagi K, Matsumoto N, Okazaki J, Kawazoe T (2020) Decontamination of titanium surface using different methods: an in vitro study. Materials (Basel) 13(10):2287. https://doi.org/10.3390/ma13102287
Yang SM, Park JB, Ko Y (2015) Use of confocal microscopy for quantification of plastic remnants on rough titanium after instrumentation and evaluation of efficacy of removal. Int J Oral Maxillofac Implants 30(3):519–525. https://doi.org/10.11607/jomi.3500
Acknowledgements
We appreciate Ricarda Albert and Lubow Woronina for support in collecting the data.
Funding
The study was financed by Zyfoma GmbH, Weiterstadt, Germany. The authors Schlee and Zipprich declare to hold patents on the described technology and to have financial interests.
Author information
Authors and Affiliations
Contributions
All authors significantly contributed to the realization of the manuscript and approved the last version before submission. HZ: research design, experiments, data acquisition, finalizing the writing, approval for submission. PW: research design, experiments, finalizing the writing, approval for submission. RG: analysis and interpretation of data, drafting and finalizing the writing, approval for submission. LS: analysis and interpretation of data, drafting and finalizing the writing, approval for submission. DH: research design, experiments, finalizing the writing, approval for submission. HLW: analysis and interpretation of data, finalizing the writing, approval for submission. MS: research design, experiments, data acquisition, finalizing the writing, approval for submission. CR: research design, experiments, data acquisition, finalizing the writing, approval for submission.
Corresponding author
Ethics declarations
Ethics approval
Ethics approval was not required for this in vitro study.
Conflict of interest
HZ and MS hold patent for the described technology and received funding by Zyfoma GmbH (Weiterstadt, Germany) for the realization of the study. All other authors reported no conflict of interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zipprich, H., Weigl, P., Di Gianfilippo, R. et al. Comparison of decontamination efficacy of two electrolyte cleaning methods to diode laser, plasma, and air-abrasive devices. Clin Oral Invest 26, 4549–4558 (2022). https://doi.org/10.1007/s00784-022-04421-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-022-04421-0