Abstract
Objectives
To evaluate the effect of non-viral gene therapy on human dental pulp stem cells (DPSCs) in an in vitro and an ex vivo model.
Materials and methods
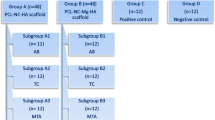
Nanoplexes comprising polyethyleneimine (PEI) and plasmid DNA (pDNA) encoding for fibroblast growth factor-2 (pFGF-2) and bone morphogenic protein-2 (pBMP-2) were cultured with DPSCs to evaluate cytotoxicity, protein expression, and mineralization activity. Collagen scaffolds loaded with these nanoplexes or mineral trioxide aggregate (MTA) were utilized in an ex vivo tooth culture model to assess pulp response, over a period of 14 days. All nanoplex formulations were characterized for size and zeta potential by measuring dynamic light scattering and electrophoretic mobility, respectively.
Results
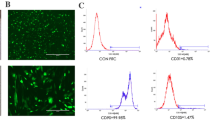
DPSCs treated with the nanoplexes showed increased cell proliferation and enhanced expression of BMP-2 and FGF-2 proteins. Collagen scaffolds containing PEI-pBMP-2 and/or pFGF-2 nanoplexes significantly increased cell proliferation, BMP-2 and FGF-2 expression, and mineralization when compared to MTA. Ex vivo histology showed a well-preserved pulp and healthy tissue in both the MTA and scaffold groups. Connective tissue in contact with the scaffold was dense and homogeneous, with some cells present in contact and within the scaffold.
Conclusion
Transfection of DPSCs with pBMP-2/pFGF-2 nanoplexes resulted in increased expression of BMP-2 and FGF-2, enhanced proliferation, and mineralization properties compared to MTA. These findings were supported by the ex vivo observations.
Clinical relevance
This biological approach in pulp capping brings new insights into the effective management of engineered pulp tissues, mainly those generated by the transplantation of DPSCs in empty root canals.




Similar content being viewed by others
References
Witherspoon DE (2008) Vital pulp therapy with new materials: new directions and treatment perspectives—permanent teeth. Pediatr Dent 30:220–224
Lanza R, Langer R, Vacanti J (2014) Principles of tissue engineering. Academic Press, San Diego
Diogenes A, Hargreaves KM (2017) Microbial modulation of stem cells and future directions in regenerative endodontics. J Endod 43:S95–S101
Nakashima M, Iohara K (2017) Recent progress in translation from bench to a pilot clinical study on total pulp regeneration. J Endod 43:S82–S86
Cavalcanti BN and Nör JE (2019) Current and future views on pulpal tissue engineering. Book title. Springer
Graham L, Cooper PR, Cassidy N, Nor JE, Sloan AJ, Smith AJ (2006) The effect of calcium hydroxide on solubilisation of bio-active dentine matrix components. Biomaterials 27:2865–2873
Murray PE, Windsor LJ, Smyth TW, Hafez AA, Cox CF (2002) Analysis of pulpal reactions to restorative procedures, materials, pulp capping, and future therapies. Crit Rev Oral Biol Med 13:509–520
Yildirim S, Can A, Arican M, Embree MC, Mao JJ (2011) Characterization of dental pulp defect and repair in a canine model. Am J Dent 24:331
Li Z, Cao L, Fan M, Xu Q (2015) Direct pulp capping with calcium hydroxide or mineral trioxide aggregate: a meta-analysis. J Endod 41:1412–1417
Tomson P, Lumley P, Smith A, Cooper P (2017) Growth factor release from dentine matrix by pulp-capping agents promotes pulp tissue repair-associated events. Int Endod J 50:281–292
Torabinejad M, Parirokh M, Dummer P (2018) Mineral trioxide aggregate and other bioactive endodontic cements: an updated overview–part II: other clinical applications and complications. Int Endod J 51:284–317
Nakashima M, Reddi AH (2003) The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol 21:1025–1032
Bakopoulou A, Papachristou E, Bousnaki M, Hadjichristou C, Kontonasaki E, Theocharidou A, Papadopoulou L, Kantiranis N, Zachariadis G, Leyhausen G (2016) Human treated dentin matrices combined with Zn-doped, mg-based bioceramic scaffolds and human dental pulp stem cells towards targeted dentin regeneration. Dent Mater 32:e159–e175
Atluri K, Seabold D, Hong L, Elangovan S, Salem AK (2015) Nanoplex-mediated codelivery of fibroblast growth factor and bone morphogenetic protein genes promotes osteogenesis in human adipocyte-derived mesenchymal stem cells. Mol Pharm 12:3032–3042
Khorsand B, Nicholson N, Do A-V, Femino JE, Martin JA, Petersen E, Guetschow B, Fredericks DC, Salem AK (2017) Regeneration of bone using nanoplex delivery of FGF-2 and BMP-2 genes in diaphyseal long bone radial defects in a diabetic rabbit model. J Control Release 248:53–59
McMillan A, Nguyen MK, Gonzalez-Fernandez T, Ge P, Yu X, Murphy WL, Kelly DJ, Alsberg E (2018) Dual non-viral gene delivery from microparticles within 3D high-density stem cell constructs for enhanced bone tissue engineering. Biomaterials 161:240–255
Cleland JL, Daugherty A, Mrsny R (2001) Emerging protein delivery methods. Curr Opin Biotechnol 12:212–219
D'Mello S, Salem AK, Hong L, Elangovan S (2016) Characterization and evaluation of the efficacy of cationic complex mediated plasmid DNA delivery in human embryonic palatal mesenchyme cells. J Tissue Eng Regen Med 10:927–937
Téclès O, Laurent P, Aubut V, About I (2008) Human tooth culture: a study model for reparative dentinogenesis and direct pulp capping materials biocompatibility. J Biomed Mater Res B 85:180–187
Breunig M, Lungwitz U, Liebl R, Goepferich A (2007) Breaking up the correlation between efficacy and toxicity for nonviral gene delivery. Proc Natl Acad Sci 104:14454–14459
Yamano S, Dai J, Moursi AM (2010) Comparison of transfection efficiency of nonviral gene transfer reagents. Mol Biotechnol 46:287–300
Jaidev L, Chatterjee K (2019) Surface functionalization of 3D printed polymer scaffolds to augment stem cell response. Mater Des 161:44–54
Kumar S, Raj S, Sarkar K, Chatterjee K (2016) Engineering a multi-biofunctional composite using poly (ethylenimine) decorated graphene oxide for bone tissue regeneration. Nanoscale 8:6820–6836
Jaidev L, Chellappan DR, Bhavsar DV, Ranganathan R, Sivanantham B, Subramanian A, Sharma U, Jagannathan NR, Krishnan UM, Sethuraman S (2017) Multi-functional nanoparticles as theranostic agents for the treatment & imaging of pancreatic cancer. Acta Biomater 49:422–433
Oliveira R, Tavares W, Reis A, Silva V, Vieira L, Ribeiro Sobrinho A (2018) Cytokine expression in response to root repair agents. Int Endod J 51:1253–1260
Chmilewsky F, Jeanneau C, Laurent P, About I (2014) Pulp fibroblasts synthesize functional complement proteins involved in initiating dentin–pulp regeneration. Am J Pathol 184:1991–2000
de Souza Costa CA, Duarte PT, de Souza PP, Giro EM, Hebling J (2008) Cytotoxic effects and pulpal response caused by a mineral trioxide aggregate formulation and calcium hydroxide. Am J Dent 21:255–261
Acknowledgments
The authors thank the American Association of Endodontics–Foundation for Endodontics for the financial support in the competitive research grant category. Aliasger Salem acknowledges the Lyle and Sharon Bighley Professorship for the Bighley Chair.
Funding
This study was conducted with the financial support from the American Association for Endodontics Foundation, in the competitive research grant submission.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The research protocol (no. 201711716) was submitted to the University of Iowa Institutional Review Board, which determined that it did not meet the regulatory definition of human subject research thus not requiring review.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chakka, L.R.J., Vislisel, J., Vidal, C.d.P. et al. Application of BMP-2/FGF-2 gene–activated scaffolds for dental pulp capping. Clin Oral Invest 24, 4427–4437 (2020). https://doi.org/10.1007/s00784-020-03308-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03308-2