Abstract
Objectives
To compare the microbial load and composition and to determine the lipopolysaccharides (LPS) and lipoteichoic acid (LTA) concentrations found in primary apical periodontitis (PAP) and post-treatment apical periodontitis (PTAP), correlating these findings with clinical/tomographic features.
Material and methods
Sixty patients with PAP (31) and PTAP (29) were submitted to clinical and tomographic assessment. Samples were collected from each root canal using paper points for microbiological assessment (culture technique and Checkerboard DNA-DNA hybridization) and determination of LPS and LTA levels (limulus amebocyte lysate and enzyme-linked immunosorbent assays, respectively). Data were correlated with clinical/tomographic findings and statistically analyzed using the Mann-Whitney and Pearson correlation tests (α = 5%).
Results
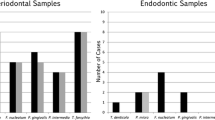
A higher number of cultivable bacteria and LPS were found in PAP (p < 0.05). The median number of species per root canal found in PAP and PTAP was 9 and 22, respectively (p < 0.05). LPS was positively correlated with a larger periapical lesion volume (p < .05). LTA levels were similar in both infections and had no correlation with signs and symptoms. In PAP, gram-positive bacteria were correlated with spontaneous pain (p < .05) and exudate (p < .05). Tenderness to percussion and pain on palpation were correlated to the presence of both gram-positive and negative bacteria. In PTAP, a positive correlation was observed between both gram-positive and gram-negative bacteria with exudate and periapical lesion volume (p < .05).
Conclusions
PAP had higher contents of microbial load and LPS compared with PTAP. However, PTAP presented a more diverse microbiota compared with PAP. Higher content of LPS was positively correlated with larger periapical bone destruction, whereas signs and symptoms with specific microorganisms.
Clinical relevance
It was verified that PAP and PTAP are polymicrobial infections with predominance of gram-negative bacteria and a more diverse bacterial population found in PTAP. A wide interaction of specific microbial species resulted in different clinical features in both infections.

Similar content being viewed by others
References
Francisco PA, Delboni MG, Lima AR, Xiao Y, Siqueira WL, Gomes BPFA (2018) Proteomic profile of root canal contents in teeth with post-treatment endodontic disease. Int Endod J. https://doi.org/10.1111/iej.13021
Cavalli D, Toia CC, Flores Orozco EI, Khoury RD, Cardoso FG d R, Alves MC, CAT C, Valera MC (2017) Effectiveness in the removal of endotoxins and microbiological profile in primary endodontic infections using 3 different instrumentation systems: a randomized clinical study. J Endod 43:1237–1245. https://doi.org/10.1016/j.joen.2017.03.032
Chugal NM, Clive JM, Spångberg LSW (2001) A prognostic model for assessment of the outcome of endodontic treatment: effect of biologic and diagnostic variables. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 91:342–352. https://doi.org/10.1067/moe.2001.113106
Chávez de Paz LE, Bergenholtz G, Svensäter G (2010) The effects of antimicrobials on endodontic biofilm bacteria. J Endod 36:70–77. https://doi.org/10.1016/j.joen.2009.09.017
Ng Y-L, Mann V, Gulabivala K (2011) A prospective study of the factors affecting outcomes of nonsurgical root canal treatment: part 1: periapical health. Int Endod J 44:583–609. https://doi.org/10.1111/j.1365-2591.2011.01872.x
Gomes BPFA, Pinheiro ET, Gadê-Neto CR, Sousa ELR, Ferraz CCR, Zaia AA, Teixeira FB, Souza-Filho FJ (2004) Microbiological examination of infected dental root canals. Oral Microbiol Immunol 19:71–76
Siqueira JF Jr, Rôças IN (2007) Bacterial pathogenesis and mediators in apical periodontitis. Braz Dent J 18:267–280. https://doi.org/10.1590/S0103-64402007000400001
Rietschel ET, Brade H (1992) Bacterial endotoxins. Sci Am 267:54–61
Martinho FC, Chiesa WMM, Leite FRM, Cirelli JA, Gomes BPFA (2011) Antigenicity of primary endodontic infection against macrophages by the levels of PGE(2) production. J Endod 37:602–607. https://doi.org/10.1016/j.joen.2010.12.005
Cardoso FGR, Ferreira NS, Martinho FC, Nascimento GG, Manhães LRC, Rocco MA, Carvalho CAT, Valera MC (2015) Correlation between volume of apical periodontitis determined by cone-beam computed tomography analysis and endotoxin levels found in primary root canal infection. J Endod 41:1015–1019. https://doi.org/10.1016/j.joen.2015.02.005
Morath S, Geyer A, Spreitzer I, Hermann C, Hartung T (2002) Structural decomposition and heterogeneity of commercial lipoteichoic acid preparations. Infect Immun 70:938–944
Gao JJ, Xue Q, Zuvanich EG, Haghi KR, Morrison DC (2001) Commercial preparations of lipoteichoic acid contain endotoxin that contributes to activation of mouse macrophages in vitro. Infect Immun 69:751–757. https://doi.org/10.1128/IAI.69.2.751-757.2001
Speers AM, Cologgi DL, Reguera G (2009) Anaerobic cell culture. In: Current protocols in microbiology. Wiley, Hoboken, p appendix 4F
Petti CA, Polage CR, Schreckenberger P (2005) The role of 16S rRNA gene sequencing in identification of microorganisms misidentified by conventional methods. J Clin Microbiol 43:6123–6125. https://doi.org/10.1128/JCM.43.12.6123-6125.2005
Ferreira NS, Martinho FC, Cardoso FGR, Nascimento GG, Carvalho CAT, Valera MC (2015) Microbiological profile resistant to different intracanal medications in primary endodontic infections. J Endod 41:824–830. https://doi.org/10.1016/j.joen.2015.01.031
Möller AJ (1966) Microbiological examination of root canals and periapical tissues of human teeth. Methodological studies. Odontol Tidskr 74(Suppl):1–380
Socransky SS, Haffajee AD, Smith C, Martin L, Haffajee JA, Uzel NG, Goodson JM (2004) Use of checkerboard DNA-DNA hybridization to study complex microbial ecosystems. Oral Microbiol Immunol 19:352–362. https://doi.org/10.1111/j.1399-302x.2004.00168.x
Blome B, Braun A, Sobarzo V, Jepsen S (2008) Molecular identification and quantification of bacteria from endodontic infections using real-time polymerase chain reaction. Oral Microbiol Immunol 23:384–390. https://doi.org/10.1111/j.1399-302X.2008.00440.x
Cogulu D, Uzel A, Oncag O, Aksoy SC, Eronat C (2007) Detection of enterococcus faecalis in necrotic teeth root canals by culture and polymerase chain reaction methods. Eur J Dent 1:216–221
Schirrmeister JF, Liebenow A-L, Braun G, Wittmer A, Hellwig E, Al-Ahmad A (2007) Detection and eradication of microorganisms in root-filled teeth associated with periradicular lesions: an in vivo study. J Endod 33:536–540. https://doi.org/10.1016/j.joen.2007.01.012
Schirrmeister JF, Liebenow A-L, Pelz K, Wittmer A, Serr A, Hellwig E, Al-Ahmad A (2009) New bacterial compositions in root-filled teeth with periradicular lesions. J Endod 35:169–174. https://doi.org/10.1016/j.joen.2008.10.024
Akpata ES (1976) Effect of endodontic procedures on the population of viable microorganisms in the infected root canal. J Endod 2:369–373. https://doi.org/10.1016/S0099-2399(76)80099-4
Sathorn C, Parashos P, Messer H (2007) How useful is root canal culturing in predicting treatment outcome? J Endod 33:220–225. https://doi.org/10.1016/j.joen.2006.11.006
Gomes BP, Drucker DB, Lilley JD (1994) Associations of specific bacteria with some endodontic signs and symptoms. Int Endod J 27:291–298
Ahn SH, Song J-E, Kim S, Cho S-H, Lim YK, Kook J-K, Kook M-S, Lee T-H (2016) NOX1/2 activation in human gingival fibroblasts by Fusobacterium nucleatum facilitates attachment of Porphyromonas gingivalis. Arch Microbiol 198:573–583. https://doi.org/10.1007/s00203-016-1223-7
Enersen M, Nakano K, Amano A (2013) Porphyromonas gingivalis fimbriae. J Oral Microbiol 5:20265. https://doi.org/10.3402/jom.v5i0.20265
Li X, Kolltveit KM, Tronstad L, Olsen I (2000) Systemic diseases caused by oral infection. Clin Microbiol Rev 13:547–558
Stuart C, Schwartz S, Beeson T, Owatz C (2006) Enterococcus faecalis: its role in root canal treatment failure and current concepts in retreatment. J Endod 32:93–98. https://doi.org/10.1016/j.joen.2005.10.049
Zoletti GO, Pereira EM, Schuenck RP, Teixeira LM, Siqueira JF, dos Santos KRN (2011) Characterization of virulence factors and clonal diversity of Enterococcus faecalis isolates from treated dental root canals. Res Microbiol 162:151–158. https://doi.org/10.1016/j.resmic.2010.09.018
Gomes BPFA, Pinheiro ET, Jacinto RC, Zaia AA, Ferraz CCR, Souza-Filho FJ (2008) Microbial analysis of canals of root-filled teeth with periapical lesions using polymerase chain reaction. J Endod 34:537–540. https://doi.org/10.1016/j.joen.2008.01.016
Gomes BPFA, Pinheiro ET, Sousa ELR, Jacinto RC, Zaia AA, Ferraz CCR, de Souza-Filho FJ (2006) Enterococcus faecalis in dental root canals detected by culture and by polymerase chain reaction analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 102:247–253. https://doi.org/10.1016/j.tripleo.2005.11.031
Pourhajibagher M, Bahador A (2018) Diagnostic accuracy of multiplex real-time PCR approaches compared with cultivation -based detection methods: monitoring the endopathogenic microbiota pre and post photo-activated disinfection. Photodiagn Photodyn Ther 22:140–146. https://doi.org/10.1016/j.pdpdt.2018.03.003
Sjogren U, Hagglund B, Sundqvist G, Wing K (1990) Factors affecting the long-term results of endodontic treatment. J Endod 16:498–504. https://doi.org/10.1016/S0099-2399(07)80180-4
Pratt I, Aminoshariae A, Montagnese TA, Williams KA, Khalighinejad N, Mickel A (2016) Eight-year retrospective study of the critical time lapse between root canal completion and crown placement: its influence on the survival of endodontically treated teeth. J Endod 42:1598–1603. https://doi.org/10.1016/j.joen.2016.08.006
Pai SF, Yang SF, Sue WL, Chueh LH, Rivera EM (1999) Microleakage between endodontic temporary restorative materials placed at different times. J Endod 25:453–456. https://doi.org/10.1016/S0099-2399(99)80278-7
Tennert C, Eismann M, Goetz F, Woelber JP, Hellwig E, Polydorou O (2015) A temporary filling material used for coronal sealing during endodontic treatment may cause tooth fractures in large class II cavities in vitro. Int Endod J 48:84–88. https://doi.org/10.1111/iej.12280
Siqueira JF, Rôças IN, Souto R, de Uzeda M, Colombo AP (2000) Checkerboard DNA-DNA hybridization analysis of endodontic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 89:744–748
Baumgartner JC (2004) Microbiological and molecular analysis of endodontic infections. Endod Top 7:35–51. https://doi.org/10.1111/j.1601-1546.2004.00061.x
Anderson AC, Hellwig E, Vespermann R, Wittmer A, Schmid M, Karygianni L, Al-Ahmad A (2012) Comprehensive analysis of secondary dental root canal infections: a combination of culture and culture-independent approaches reveals new insights. PLoS One 7:e49576. https://doi.org/10.1371/journal.pone.0049576
Baumgartner J, Siqueira JRJ, Xia T, Roças I (2004) Geographical differences in bacteria detected in endodontic infections using polymerase chain reaction. J Endod 30:141–144. https://doi.org/10.1097/00004770-200403000-00004
Harbarth S, Samore MH (2005) Antimicrobial resistance determinants and future control. Emerg Infect Dis 11:794–801. https://doi.org/10.3201/eid1106.050167
Gomes BPFA, Endo MS, Martinho FC (2012) Comparison of endotoxin levels found in primary and secondary endodontic infections. J Endod 38:1082–1086. https://doi.org/10.1016/j.joen.2012.04.021
Yang J, Park O-J, Kim J, Baik JE, Yun C-H, Han SH (2016) Lipoteichoic acid of Enterococcus faecalis inhibits the differentiation of macrophages into osteoclasts. J Endod 42:570–574. https://doi.org/10.1016/j.joen.2016.01.012
Mörmann M, Thederan M, Nackchbandi I, Giese T, Wagner C, Hänsch GM (2008) Lipopolysaccharides (LPS) induce the differentiation of human monocytes to osteoclasts in a tumour necrosis factor (TNF) α-dependent manner: a link between infection and pathological bone resorption. Mol Immunol 45:3330–3337. https://doi.org/10.1016/j.molimm.2008.04.022
Boyce BF, Xing L (2008) Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys 473:139–146. https://doi.org/10.1016/j.abb.2008.03.018
Funding
The work was supported by the Programa Nacional de Cooperação Acadêmica da Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–CAPES/Brasil and the Fundação de Amparo à Pesquisa do Estado de São Paulo-FAPESP (grant numbers. 2015/05397-1 and 2018/01703-9).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the local Institute Review Board (São Paulo State University (Unesp), Institute of Science and Technology, São José dos Campos, Brazil) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Machado, F.P., Khoury, R.D., Toia, C.C. et al. Primary versus post-treatment apical periodontitis: microbial composition, lipopolysaccharides and lipoteichoic acid levels, signs and symptoms. Clin Oral Invest 24, 3169–3179 (2020). https://doi.org/10.1007/s00784-019-03191-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-03191-6