Abstract
Objectives
To investigate the effects of low-level laser irradiation (LLLI) on viability/proliferation, migration, osteo/odontogenic differentiation, and in vitro biomineralization of stem cells from human exfoliated deciduous teeth (SHED).
Materials and methods
SHED cultures were established by enzymatic dissociation from pulps of deciduous teeth. SHED were irradiated with a diode laser (InGaAsP; 940 nm; 0.2 W, continuous mode) at energy fluences 4, 8, and 16 J/cm2 in the dark, while non-irradiated SHED served as control. Cell viability/proliferation was evaluated by MTT assay and cell mobilization by Transwell™ migration assay. Expression of osteo/odontogenesis-related genes (ALP, BMP-2, BGLAP, DSPP, MSX2, RUNX2) was assessed by real-time PCR, while in vitro biomineralization by Alizarin Red staining. Statistical analysis was performed by two-way ANOVA and Tukey’s post hoc tests (*p < 0.05, **p < 0.01).
Results
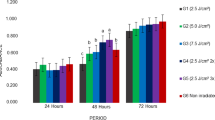
Statistically significant stimulation of cell viability/proliferation was observed at all energy fluences, reaching the highest effect for the 4 and 16 J/cm2. Although the 8 J/cm2 fluence showed the lowest stimulatory effect on cell viability/proliferation, it was the most effective in inducing SHED migration, upregulation of odontogenesis-related genes (DSPP, ALP, BMP-2) at specific time-points, and the in vitro biomineralization potential of SHED compared to the other two energy fluences.
Conclusions
LLLI proved beneficial in promoting SHED biological processes critical for pulp repair in deciduous teeth. Overall, the 8 J/cm2 energy fluence showed the most beneficiary effects.
Clinical relevance
These results provide insights on a narrow “therapeutic window” of LLLI application in vital pulp therapies of deciduous teeth, paving the way for the establishment of effective clinical protocols.





Similar content being viewed by others
References
Coll JA, Seale NS, Vargas K, Marghalani AA, Al Shamali S, Graham L (2017) Primary tooth vital pulp therapy: a systematic review and meta-analysis. Pediatr Dent 39(1):16–123
Dhar V, Marghalani AA, Crystal YO, Kumar A, Ritwik P, Tulunoglu O, Graham L (2017) Use of vital pulp therapies in primary teeth with deep caries lesions. Pediatr Dent 39(5):146–159
Fuks AB, Guelmann M, Kupietzky RI, Costa CAS (2012) Current developments in pulp therapy for primary teeth. Endod Top 23:50–72. https://doi.org/10.1111/etp.12003
Olivi G, Margolis F, Genovese MD (2011) Basic science of laser dentistry in: pediatric laser dentistry, a user’s guide, 1st edn. Quintessence Pub Co, Inc, Chicago, pp 3–13
Arapostathis K (2017) Laser-assisted pediatric dentistry. In: Coluzzi DJ, Parker SA (eds) Lasers in dentistry—current concepts, 1st edn. Springer, Cham, pp 235–238. https://doi.org/10.1007/978-3-319-51944-9
Convissar RA, Tunér J, Beck-Kristensen PH, Ross G, Ross A (2016) Photobiomodulation in dentistry. Princ Pract Laser Dent:251–274. https://doi.org/10.1016/B978-0-323-29762-2.00015-2
Romagnoli E, Cafaro A (2017) PBM. Theoretical and applied concepts of adjunctive use of LLLT/PBM within clinical dentistry. In: Coluzzi DJ, Parker SA (eds) Lasers in dentistry—current concepts. Springer, Cham, pp 135–140. https://doi.org/10.1007/978-3-319-51944-9_11
Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR (2012) The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng 40(2):516–533. https://doi.org/10.1007/s10439-011-0454-7
Kushibiki T, Hirasawa T, Okawa S, Ishihara M (2013) Regulation of miRNA expression by low-level laser therapy (LLLT) and photodynamic therapy (PDT). Int J Mol Sci Int J Mol Sci 14:13542–13558
Montoro LA, Turrioni AP, Basso FG, de Souza Costa CA, Hebling J (2014) Infrared LED irradiation photobiomodulation of oxidative stress in human dental pulp cells. Int Endod J 47(8):747–755. https://doi.org/10.1111/iej.12211
Barolet D (2008) Light-emitting diodes (LEDs) in dermatology. Semin Cutan Med Surg 27(4):227–238. https://doi.org/10.1016/j.sder.2008.08.003
Ginani F, Soares DM, Augusto C, Barboza G (2015) Effect of low-level laser therapy on mesenchymal stem cell proliferation: a systematic review. Lasers Med Sci 30(8):2189–2194. https://doi.org/10.1007/s10103-015-1730-9
Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S (2003) SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A 100:5807–5812
Kashyap R (2015) SHED—basic structure for stem cell research. J Clin Diagn Res 9(3):ZE07–ZE09. https://doi.org/10.7860/JCDR/2015/9871.5636
Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith AJ, Nör JE (2008) Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod 34:962–969. https://doi.org/10.1016/j.joen.2008.04.009
Golpayegani MV, Ansari G, Tadayon N, Shams S, Mir M (2009) Low-level laser therapy for pulpotomy treatment of primary molars. J Dent 6(4):168–174
Uloopi KS, Vinay C, Ratnaditya A, Gopal AS, Mrudula KJ, Rao RC (2016) Clinical evaluation of low level diode laser application for primary teeth pulpotomy. J Clin Diagn Res: J Clin Diagn Res 10(1):ZC67–ZC70. https://doi.org/10.7860/JCDR/2016/13218.7140
Olivi G, Magnolis FS, Genovese MD (2011) Endodontics. In: Pediatric laser dentistry, a user’s guide, 1st edn. Quintessence Pub Co, Inc, Chicago, p 93
Bidar M, Moushekhian S, Gharechahi M, Talati A, Ahrari F, Bojarpour M (2016) The effect of low level laser therapy on direct pulp capping in dogs. J Lasers Med Sci 7(3):177–183. https://doi.org/10.15171/jlms.2016.31
Turrioni AP, Basso FG, Montoro LA, Almeida Lde F, Costa CA, Hebling J (2014) Phototherapy up-regulates dentin matrix proteins expression and synthesis by stem cells from human-exfoliated deciduous teeth. J Dent 42(10):1292–1299. https://doi.org/10.1016/j.jdent.2014.07.014
Turrioni AP, Montoro LA, Basso FG, de Almeida Lde F, Costa CA, Hebling J (2015) Dose-responses of stem cells from Hyman exfoliated teeth to infrared LED irradiation. Braz Dent J 26:409–415. https://doi.org/10.1590/0103-6440201300148
Turrioni AP, Basso FG, Montoro LA, Almeida LFD, de Souza Costa CA, Hebling J (2016) Transdentinal photobiostimulation of stem cells from human exfoliated primary teeth. Int Endod J 50:549–559
Diniz IMA, Matos AB, Marques MM (2015) Laser phototherapy enhances mesenchymal stem cells survival in response to the dental adhesives. SciWorld J 2015:671789. https://doi.org/10.1155/2015/671789
Fernandes AP, Junqueira MA, Marques NCT, Machado MAAM, Santos CF, Oliveira TM, Sakai VT (2016) Effects of low-level laser therapy on stem cells from human exfoliated deciduous teeth. J Appl Oral Sci 24(4):332–337. https://doi.org/10.1590/1678-775720150275
Ginani F, Soares DM, de Oliveira Rocha HA, de Souza LB, Barboza CG (2018) Low-level laser irradiation induces in vitro proliferation of stem cells from human exfoliated deciduous teeth. Lasers Med Sci 33:95–102
Bakopoulou A, Leyhausen G, Volk J, Tsiftsoglou A, Garefis P, Koidis P, Geurtsen W (2011) Assessment of the impact of two different isolation methods on the Osteo/odontogenic differentiation potential of human dental stem cells derived from deciduous teeth. Calcif Tissue Int 88:130–141. https://doi.org/10.1007/s00223-010-9438-0
Bakopoulou A, Papachristou E, Bousnaki M, Hadjichristou C, Kontonasaki E, Theocharidou A, Papadopoulou L, Kantiranis N, Zachariadis G, Leyhausen G, Geurtsen W, Koidis P (2016) Human treated dentin matrices combined with Zn-doped, mg-based bioceramic scaffolds and human dental pulp stem cells towards targeted dentin regeneration. Dent Mater 32:e159–e175. https://doi.org/10.1016/j.dental.2016.05.013
Salzig D, Leber J, Merkewitz K, Lange MC, Köster N, Czermak P (2016) Attachment, growth, and detachment of human mesenchymal stem cells in a chemically defined medium. Stem Cells Int 5246584. https://doi.org/10.1155/2016/5246584
Tuby H, Maltz L, Oron U (2007) Low-level laser irradiation (LLLI) promotes proliferation of mesenchymal and cardiac stem cells in culture. Lasers Surg Med 39:373–378. https://doi.org/10.1002/lsm.20492
Zaccara IM, Ginani F, Mota-Filho HG, Henriques ÁG, Barboza CG (2015) Effect of low-level laser irradiation on proliferation and viability of human dental pulp stem cells. Lasers Med Sci 30:2259–2264
Amid R, Kadkhodazadeh M, Ahsaie MG, Hakakzadeh A (2014) Effect of low level laser therapy on proliferation and differentiation of the cells contributing in bone regeneration. J Lasers Med Sci 5:163–170
AlGhamdi KM, Kumar A, Moussa NA (2012) Low-level laser therapy: a useful technique for enhancing the proliferation of various cultured cells. Lasers Med Sci 27(1):237–249. https://doi.org/10.1007/s10103-011-0885-2
Barboza CA, Ginani F, Soares DM, Henriques AC, Freitas Rde A (2014) Low-level laser irradiation induces in vitro proliferation of mesenchymal stem cells. Einstein (Sao Paulo) 12(1):75–81. https://doi.org/10.1590/0S1679-45082014AO2824
Borzabadi-Farahani A (2016) Effect of low-level laser irradiation on proliferation of human dental mesenchymal stem cells; a systemic review. J Photochem Photobiol B Biol 162:577–582
Li W, Chen C, Huang P (2013) Effects of low level light irradiation on the migration of mesenchymal stem cells derived from rat bone marrow. Conf Proc IEEE Eng Med Biol Soc 2013:4121–4124. https://doi.org/10.1109/EMBC.2013.6610452
Kim H, Choi K, Kweon OK, Kim WH (2012) Enhanced wound healing effect of canine adipose-derived mesenchymal stem cells with low-level laser therapy in athymic mice. J Dermatol Sci 68:149–156. https://doi.org/10.1016/j.jdermsci.2012.09.013
Shingyochi Y, Kanazawa S, Tajima S, Tanaka R, Mizuno H, Tobita M (2017) A low-level carbon dioxide laser promotes fibroblast proliferation and migration through activation of Akt, ERK, and JNK. PLoS One 12(1):e0168937. https://doi.org/10.1371/journal.pone.0168937
Andrade Fdo S, Clark RM, Ferreira ML (2014) Effects of low-level laser therapy on wound healing. Rev Col Bras Cir 41(2):129–133. https://doi.org/10.1590/S0100-69912014000200010
Park BW, Hah YS, Choi MJ, Ryu YM, Lee SG, Kim DR, Kim JR, Byun JH (2009) In vitro osteogenic differentiation of cultured human dental papilla-derived cells. J Oral Maxillofac Surg 67(3):507–514. https://doi.org/10.1016/j.joms.2008.08.037
Papagerakis P, Berdal A, Mesbah M, Peuchmaur M, Malaval L, Nydegger J, Simmer J, Macdougall M (2002) Investigation of osteocalcin, osteonectin, and dentin sialophosphoprotein in developing human teeth. Bone 30(2):377–385
Casagrande L, Cordeiro MM, Nör SA, Nör JE (2011) Dental pulp stem cells in regenerative dentistry. Odontology 99(1):1–7. https://doi.org/10.1007/s10266-010-0154-z
Yang W, Harris MA, Cui Y, Mishina Y, Harris SE (2012) Bmp2 is required for odontoblast differentiation and pulp. J Dent Res 91(1):58–64. https://doi.org/10.1177/0022034511424409
Manzano-Moreno FJ, Medina-Huertas R, Ramos-Torrecillas J, García-Martínez O, Ruiz C (2015) The effect of low-level diode laser therapy on early differentiation of osteoblast via BMP-2/TGF-β1 and its receptors. J Cranio-Maxillofacial Surg 43:1926–1932
Fujimoto K, Kiyosaki T, Mitsui N, Mayahara K, Omasa S, Suzuki N, Shimizu N (2010) Low-intensity laser irradiation stimulates mineralization via increased BMPs in MC3T3-E1 cells. Lasers Surg Med 42(6):519–526. https://doi.org/10.1002/lsm.20880
Theocharidou A, Bakopoulou A, Kontonasaki E, Papachristou E, Hadjichristou C, Bousnaki M, Theodorou G, Papadopoulou L, Kantiranis N, Paraskevopoulos K, Koidis P (2017) Odontogenic differentiation and biomineralization potential of dental pulp stem cells inside Mg-based bioceramic scaffolds under low-level laser treatment. Lasers Med Sci 32:201–210
Komori T (2009) Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res 339(1):189–195. https://doi.org/10.1007/s00441-009-0832-8
Pyo SJ, Song WW, Kim IR, Park BS, Kim CH, Shin SH, Chung IK, Kim YD (2013) Low-level laser therapy induces the expressions of BMP-2, osteocalcin, and TGF-β1 in hypoxic-cultured human osteoblasts. Lasers Med Sci 28:543–550. https://doi.org/10.1007/s10103-012-1109-0
Bozkurt SB, Hakki EE, Kayis SA, Dundar N, Hakki SS (2017) Biostimulation with diode laser positively regulates cementoblast functions, in vitro. Lasers Med Sci 32:911–919
Butler WT, Bhown M, D’Souza RN, Farach-Carson MC, Happonen RP, Schrohenloher RE, Seyer JM, Somerman MJ, Foster RA, Tomana M, Van Dijk S (1992) Isolation, characterization and immunolocalization of a 53-kDa dentin sialoprotein. Matrix 12:343–351
Stein GS, Lian JB (1992) Gene expression during development of the osteoblast phenotype: an integrated relationship of cell growth to differentiation. In: Molecular and cellular approaches to the control of proliferation and differentiation. Academic Press. ICN, USA, pp 191–192
Chen S, Gluhak-Heinrich J, Wang YH, Wu YM, Chuang HH, Chen L, Yuan GH, Dong J, Gay I, MacDougall M (2009) Runx2, osx, and dspp in tooth development. J Dent Res 88(10):904–909
Miyazaki T, Kanatani N, Rokutanda S, Yoshida C, Toyosawa S, Nakamura R, Takada S, Komori T (2008) Inhibition of terminal differentiation of odontoblasts and their trans-differentiation in to osteoblasts in Runx2 transgenic mice. Arch Histol Cytol 71:131–146
Fujii S, Fujimoto K, Goto N, Kanawa M, Kawamoto T et al (2015) Characteristic expression of MSX1, MSX2, TBX2 and ENTPD1 in dental pulp cells. Biomed Rep 3(4):566–572. https://doi.org/10.3892/br.2015.456
Bidder M, Latifi T, Towler DA (1998) Reciprocal temporospatial patterns of Msx2 and osteocalcin gene expression during murine odontogenesis. J Bone Miner Res 13:609–619
Maas R, Bei M (1997) The genetic control of early tooth development. Crit Rev Oral Biol Med 8(1):4–39
Wanet A, Arnould T, Najimi M, Renard P (2015) Connecting mitochondria metabolism, and stem cell fate. Stem Cells Dev 24:1957–1971. https://doi.org/10.1089/scd.2015.0117
Ito K, Suda T (2014) Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol 15:243–256. https://doi.org/10.1038/nrm3772
Arana-Chave VE, Massa LF (2004) Odontoblasts: the cells forming and maintaining dentine. Int J Biochem Cell Biol 36:1367–1373
Wu Y, Wang J, Gong D, Gu H, Hu S, Zhang H (2012) Effects of low-level laser irradiation on mesenchymal stem cell proliferation: a microarray analysis. Lasers Med Sci 27(2):509–519. https://doi.org/10.1007/s10103-011-0995-x
Pedroni AC, Diniz IM, Abe GL, Moreira MS, Sipert CR, Marques MM (2018) Photobiomodulation therapy and vitamin C on longevity of cell sheets of human dental pulp stem cells. J Cell Physiol 233:7026–7703
Diniz IMA, Carreira ACO, Sipert CR, Uehara CM, Moreira MSN, Freire L, Pelissari C, Kossugue PM, de Araújo DR, Sogayar MC, Marques MM (2018) Photobiomodulation of mesenchymal stem cells encapsulated in an injectable rhBMP4-loaded hydrogel directs hard tissue bioengineering. J Cell Physiol 233:4907–4918
Ballini A, Mastrangelo F, Gastaldi G, Tettamanti L, Bukvic N, Cantore S, Cocco T, Saini R, Desiate A, Gherlone E, Scacco S (2015) Osteogenic differentiation and gene expression of dental pulp stem cells under low-level laser irradiation: a good promise for tissue engineering. J Biol Regul Homeost Agents 29(4):813–822
Holder MJ, Milward MR, Palin WM, Hadis MA, Cooper PR (2012) Effects of red light-emitting diode irradiation on dental pulp cells. J Dent Res 91:961–966
Pereira S, Tettamanti M (2005) Ahimsa and alternatives: the concept of the 4thR. The CPCSEA in India (2005). ALTEX 22(1):3–6
Acknowledgements
We would like to thank Prof. E. Kriezis, Professor of Optical and Microwave Communications at the Department of Electrical and Computer Engineering, Aristotle University of Thessaloniki (A.U.Th), Greece, for his scientific advices, as well as for the supply of relevant to LLLI measurement equipment.
Funding
The work was supported by institutional funds of the Departments Pediatric Dentistry and Prosthodontics, School of Dentistry, Faculty of Health Science, Aristotle University of Thessaloniki (A.U.Th).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures performed in studies involving human biological material (dental pulp tissue from extracted deciduous teeth) were in accordance with the ethical standards of the Institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, informed consent was obtained from the parents of all individual participants (children) included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Paschalidou, M., Athanasiadou, E., Arapostathis, K. et al. Biological effects of low-level laser irradiation (LLLI) on stem cells from human exfoliated deciduous teeth (SHED). Clin Oral Invest 24, 167–180 (2020). https://doi.org/10.1007/s00784-019-02874-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-02874-4