Abstract
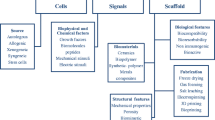
Cells, scaffold, and growth factors are crucially important in regenerative medicine and tissue engineering. Progress in science and technology has enabled development of these three factors, with basic research being applied clinically. In the past decade, we have investigated tissue regeneration in animal models of musculoskeletal disorders by using cells, scaffold, and delivery systems which has been relatively easy to apply and develop in clinical settings. Moreover, microRNA (miRNA), which are important in biological processes and in the pathogenesis of human diseases, have been used in research on regenerative medicine. For the cell source, we focused on mesenchymal stem cells (MSC) and CD34+ and CD133+ cells as endothelial progenitor cells for regeneration of musculoskeletal organs. These cells are accessible and safe. For less invasive and more effective therapy, we developed a novel cell-delivery system using magnetic force to accumulate cells at a desired site. Furthermore, administration of synthetic miRNA could enhance tissue regeneration. In our studies, use of these cells combined with a cell-delivery system, miRNA, scaffold, and cytokines has led to effective regeneration of musculoskeletal tissues including cartilage, bone, ligaments, muscle, peripheral nerves, and spinal cord. The current and future objective is more effective and less invasive cell-based therapy with spatial control of transplanted cells by use of an external magnetic force. Analysis of efficiency, safety, and the mechanism of tissue regeneration by cells, scaffold, and miRNA will lead to more promising regenerative medicine, involving the development of a new generation of therapy. This review will focus on our regenerative medicine research, which focuses on clinical application of cells, scaffold, and miRNA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2000, the WHO endorsed the “Bone and Joint Decade” to campaign for promotion of muscloskeletal health. Muscloskeletal disorders reduce the working population and, hence, increase the number of elderly people who prematurely need nursing care [1]. This is an acute problem in Japan, an example of a so-called “super-aging society”. Muscloskeletal organs consist of four components: bone, joints, muscles, and nerves. Development of cell biology and technology has enabled regeneration of these components.
Cells, scaffold, and growth factors are crucially important in regenerative medicine and tissue engineering. Progress in science and technology has enabled development of these three factors, with basic research being applied clinically; this, in turn, has improved the level of healthcare. It is important to choose appropriate cells, scaffold, and growth factors in tissue regeneration for clinical use. For the cell source, Yamanaka et al. discovered induced pluripotent stem cells (iPSC) which can differentiate into any type of cell as a result of the action of four reprogramming factors: c-Myc, Klf4, Oct3/4, and Sox2 [2]. iPSC will enable regenerative medicine to bring about radical innovative treatments for musculoskeletal disorders [3]. However, clinical application of iPSC requires more time. We have investigated tissue regeneration in musculoskeletal disorders by using autologous cells, scaffold, and a delivery system which have been used in clinical applications.
Cells
For the cell source, we focused on mesenchymal stem cell (MSC) and endothelial progenitor cell (EPC). MSC are an attractive cell source for regenerative medicine because of their ability to differentiate into osteogenic, adipogenic, and chondrogenic lineages [4]. MSC are obtained from bone marrow and separated from bone marrow aspirate on the basis of their capacity to adhere to substrates and to form colony units. MSC are characterized on the basis of surface expression markers: CD73, CD90, and CD105 positive, and CD34 and CD45 negative [5]. MSC have the capacity to migrate to an injured site by expression of receptors and adhesion molecules, and to proliferate and differentiate within repair tissue. Furthermore, MSC secrete juxtacrine or paracrine factors that enhance regeneration from host cells, including stem cells. Several reports have demonstrated that a cultured medium of MSC has the potential for tissue regeneration, because it contains exosomes which are complex, 50–200 nm in size, bi-lipid membrane vesicles [6]. Exosomes contain protein, mRNA, and miRNA. Further examination of exosomes is needed to confirm the alternative therapeutic use of MSC and to investigate the mechanism of tissue regeneration by MSC transplantation [7]. Until 1997, postnatal new vessel formation (neovascularization) was believed to be a function of “angiogenesis”, through proliferation and migration of differentiated endothelial cells, as opposed to the “vasculogenesis” process through angioblast-induced neovascularization that was believed to be restricted to embryonic development [8]. However, circulating EPC were discovered in adult peripheral blood, demonstrating the potential for differentiation into endothelial cells in vitro and neovascularization in vivo. Because of this character, emerging EPC-based cell therapy has been applied to ischemic disease, especially in the field of cardiovascular regeneration. Accumulated basic and clinical data led this strategy into the orthopaedic field, with the purpose of acceleration of tissue repair via neovascularization. Because the concept of EPC is based on formation of this cell fraction from hemangioblasts, which are closely linked to hematopoietic stem cells (HSCs), CD34 and CD133 antigens, commonly used as the surface markers of HSCs, have been reliable markers and have been used to obtain EPC on the basis of the first report. These unique CD34+ and CD133+ cells have the therapeutic potential to:
-
1.
accelerate neovascularization;
-
2.
facilitate tissue repair/regeneration; and
-
3.
modulate the regenerating environment.
Additional advantages of these cell fractions are that they can be obtained from adult peripheral blood or bone marrow without culture and then be transplanted to ischemic/damaged tissue by use of both systemic and local injections.
Cell-delivery system
For tissue regeneration it is essential to accumulate cells effectively at the injured site. Therefore, a cell-delivery system or method to accumulate cells in the desired area is required. Our cell-delivery system uses a minimally invasive external magnetic force device (Fig. 1). MSC were labeled with ferumoxides, dextran-coated superparamagnetic iron oxide nanoparticles approved by the US Food and Drug Administration as a magnetic resonance contrast agent for hepatic imaging of humans. We originally made an external magnetic device that generated a high magnetic force, and succeeded in accumulating magnetically labeled MSC (m-MSC) in the desired area [9]. For cartilage repair, adhesion of m-MSC to the cartilage defect in this novel technique was approximately 95 % and higher than in the local adhesion technique [10].Our study also confirmed that the proliferation and chondrogenic, osteogenic, and adipogenic differentiation of m-MSC were not affected by magnetic labeling and exposure to a magnetic force [10]. To enable clinical use of this cell-delivery technique, we have worked on regeneration after cartilage, bone, muscle, and spinal injury in an animal model.
microRNA
In addition to cell therapy, we have examined the potential of tissue regeneration by microRNA (miRNA) which are important in biological processes and human diseases. miRNA are a class of non-coding RNA that regulate gene expression post-transcriptionally, and are recognized as one of the major regulators of a variety of biological processes, for example the cell cycle, immune function, and metabolism [11, 12]. miRNA regulate gene expression by binding 3′ UTR of their target mRNA before translational repression or mRNA degradation. Many miRNA are evolutionarily conserved across phyla, identified from nematodes to humans. Because miRNA are of crucial importance in the pathogenesis of human diseases, including in the orthopaedic field, miRNA have attracted attention for developing a novel therapeutic strategy by targeting miRNA. Because miRNA are essential for the development and homeostasis of components of muscloskeletal organs, for example cartilage, bone, and muscle, the existence of miRNA in muscloskeletal disorder has become increasingly important. Interestingly, miRNA circulate in body fluid packaged in microvesicles, for example exosomes, and are secreted from cells, which have attracted interest for therapeutic application, for example as biomarkers [13].
Scaffold
For cell proliferation and differentiation, scaffold provides a 3-dimensional surrounding environment and growth factors provide a suitable stimulus. Atelocollagen® (Koken, Tokyo, Japan), type I collagen gel, is a suitable scaffold and carrier for tissue engineering. It is extracted from bovine calf skin by pepsin digestion, and immunogenicity and a safety problem have been solved by removing antigenic determinants from the peptide chains of type I collagen. It has been used clinically since 1986 for skin disorders and cosmetic surgery; we are, therefore, focusing on the carrier properties of atolocollagen gel. In a monolayer culture, chondrocytes assume a dedifferentiated fibroblastic morphology, with loss of the ability to accumulate a matrix and synthesize predominantly type I collagen. We evaluated the effect of the 3-dimensional culture of human chondrocytes embedded in atelocollagen gel. Chondrocytes embedded in atelocollagen gel proliferated and produced chondroitin 6-sulfate, maintaining the chondrocyte phenotype for up to 4 weeks [14]. In our study, atelocollagen was used as the carrier of cells for articular cartilage, muscle, and nerve repair. Atelocollagen has been also used as a biomaterial carrier for gene delivery in vitro and in vivo. Atelocollagen-mediated siRNA or miRNA delivery is effective for gene silencing in vivo, because atelocollagen complexed with siRNA or miRNA is resistant to nuclease and can be efficiently transduced into cells [15].
Hydroxyapatite ceramic has been used as a bone substitute because of its good biocompatibility. However, conventional hydroxyapatite ceramic has poor osteoconductive capacity because of an insufficient number of pore connections. Interconnected porous calcium hydroxyapatite ceramic (IP-CHA; NEOBONE®; MMT, Osaka, Japan) is an innovative invention because it has spherical uniform pores, and almost all pores connect through large interconnective holes [16]. These features enable the cells and tissues to penetrate the center of the IP-CHA, providing good osteoconductivity at an early stage. This feature has the additional advantage of carrying the cells or growth factors.
Growth factor/cytokine
To promote cell proliferation or production of an extracellular matrix, application of cytokines or growth factors is useful. We focused on hyaluronic acid (HA), safe clinical use of which has been confirmed. Under a 3-dimensional culture of chondrocytes, HA has the capacity to promote cell proliferation and synthesis of chondroitin sulfate [17]. Application of growth factors or modification of the environment to up-regulate growth factors or cytokine should be considered for tissue regeneration.
This review will focus on our regenerative medicine research on cells, scaffold, and miRNA in view of their clinical application.
Cartilage
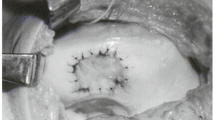
In 1994, Brittberg et al. reported successful autologous chondrocyte implantation (ACI) involving the suspension of autologous chondrocytes expanded in a monolayer culture system transplanted into a cartilage defect and covered with a periosteal flap [18]. Although this report had a big impact on cartilage repair, there were several lingering concerns, for example transplantation of dedifferentiated chondrocytes in a monolayer culture, the risk of leakage of the transplanted chondrocytes from the cartilage defects, and uneven distribution of chondrocytes in the transplanted site by gravity. To solve these problems, we used a 3-dimensional culture system with atelocollagen gel [19]. Our procedure involving tissue engineered cartilage made ex vivo then transplanted into the cartilage defect and covered with a periosteal flap has been furnishing good clinical results since 1996. However, because this transplantation procedure requires an arthrotomy we developed a tissue-engineered chondral plug [20]. Tissue engineered cartilage was placed on type I collagen sponge surrounded by PLLA mesh, and this chondral plug was successfully transplanted into the osteochondral defect of the rabbit patellar groove, producing good results. As a less invasive MSC procedure, we examined the effect of intra-articular MSC injection. The effect of the combination therapy of drilling with intra-articular injection of bone marrow MSC on the repair of a cartilage defect in a rat model was examined [21]. The results revealed that the combination of drilling with an MSC injection is better than drilling only, suggesting the efficiency of intra-articular injection of MSC. However, an intra-articular injection containing too many MSC is problematic. We demonstrated that 1 × 107 MSC generated free bodies of scar tissue in the joint after an intra-articular injection [22]. Because generation of scar tissues leads to arthrofibrosis, the optimum number of cells for tissue regeneration should be defined. To avoid this problem, a magnetic targeting system for cartilage defects has been investigated in our laboratory. To examine whether magnetically labeled cells could accumulate in the cartilage defect and enable in-vivo repair, the effect of intra-articular injection of MSC into a large animal with an external magnetic force was evaluated [10]. A full thickness cartilage defect was created in the center of the patella in mini-pigs. Four weeks after creation of the cartilage defect, magnetically labeled MSC (5 × 106 cells) were injected and were accumulated in the cartilage defect under a 1.5 T external magnetic force for 10 min (Fig. 2a). Twelve weeks after injection, arthroscopic findings revealed good integration in the border zone and the surface of the regenerated tissue was smooth, with stiffness nearly normal in the grafts. Twelve and 24 weeks after injection, histology demonstrated that the cartilage defect was repaired with hyaline-like cartilage (Fig. 2b). A small number of magnetically labeled MSC and an external magnetic force have effective potential for cartilage repair with hyaline like cartilage, leading to the development of a novel therapeutic option.
Bone
Although IP-CHA has good osteoconductivity, MSC-loaded IP-CHA would have the potential to accelerate bone formation for large bone defects. We have demonstrated excellent bone formation of MSC/IP-CHA composite in the rat tibial condyle compared with implantation of IP-CHA only [23]. In this study, implanted MSC survived 8 weeks after surgery, and differentiated into osteoclast-like cells. This hybridized IP-CHA is useful for accelerated bone formation, but a concern remains. In this hybridized technique, cells are incorporated into the IP-CHA preoperatively, but problems such as poor bone formation occur post-operatively in clinical application. To solve these problems, a novel cell-delivery system is needed to carry cells into the center of the graft site. We propose local injection of magnetically labeled MSC into the bone defect side in combination with a magnetic targeting system [24]. In our study, IP-CHA was grafted to bridge a rabbit ulnar defect, and 2 weeks later, magnetically labeled MSC were injected percutaneously into the rabbit ulnar. This external magnetic targeting system could attract cells into the center of the IP-CHA, subsequently achieving enhancement of bone formation even in the chronic phase. However, because the fate of magnetically labeled MSC was unclear, we tracked their behavior by use of an in vivo bioluminescence imaging system in the rat non-healing femoral fracture model; the results demonstrated that the external magnetic delivery system promoted cell accumulation, adhesion, and proliferation at the fracture site [25]. These results suggested the potential clinical use of magnetically labeled MSC for the treatments of bone defect, delayed union, and nonunion.
Ligaments
The healing potential of the anterior cruciate ligament (ACL) is recognized as extremely poor, because rates of cell division and migration of fibroblasts from the ACL are lower than for those from the medial collateral ligament [26]. MSC are useful for ligament healing, because of their high potential for the proliferation and collagen production. We created an ACL partially transected model in rats, and injected 1 × 106 MSC into the knee joint [27]. Four weeks after intra-articular injection, the transected area was covered with healing tissue, and the histologic score and ultimate ACL load of the MSC-injected knee was significantly better than that of the non-injected knee. These results indicated that injected MSC can accelerate the healing of a partially torn ACL. Another reason for poor healing of the ACL is that its blood supply is restricted, and angiogenesis in the injured ACL occurs very slowly. MiR-210 is known to be extremely important in angiogenesis, and is up-regulated in response to hypoxia, subsequently affecting endothelial cell survival, migration, and differentiation. Over-expression of miR-210 in endothelial cells can stimulate formation of capillary structure. We hypothesized that miR-210 administration by intra-articular injection would accelerate ACL healing via enhancement of angiogenesis [28]. Expression of miR-210 decreases after ACL transection but gradually increases after that. An intra-articular injection of an miR-210 mimic could improve the biomechanical properties of partially transected ACL, achieving good coverage of healing tissue with abandoned vessels on the injured site by up-regulation of VEGF, FGF2, and collagen type 1, suggesting the possibility of miRNA therapy for ACL injury.
Muscle
Because such complications as muscle contracture, atrophy, and residual pain are often encountered, several studies have attempted to achieve complete muscle recovery by use of drugs, cytokines and cell therapy. We examined muscle regeneration in a rat by transplantation of bone marrow-derived MSC into a muscle injured site [29]. One month after transplantation, the transplanted MSC had promoted histological and functional muscle recovery, and although the mechanism of muscle recovery acceleration (including cell fate) was not elucidated, these results suggested the usefulness of cell therapy for muscle injury. We next investigated whether human CD133+ cells have the therapeutic potential for skeletal muscle regeneration in the rat anterior tibialis muscle laceration model [30]. 1 × 105 CD133+ cells were injected locally into an injured muscle site. It was confirmed that the injected CD133+ cells differentiate into endothelial and myogenic lineages, and that they enhance the histological and functional recovery of injured muscle by inhibiting fibrosis and up-regulating angiogenesis and myogenesis. Although the transplantation of human peripheral blood CD133+ cells inhibits fibrosis and improves muscle regeneration in rat skeletal muscle injury, it is difficult to obtain a sufficient number of cells because of the small population of CD133+ cells. Our study has confirmed that accumulation of a small number of cells at an injured site by use of a magnetic cell targeting system could enhance the regenerative effect of transplantation of CD133+ cells [31]. By using a magnetic cell separation instrument, CD133+ cells were labeled with magnetic beads to isolate them from human peripheral blood. For magnetic cell targeting, the magnetic field generator was set up to adjust the peak of magnetic force gradient at the injured muscle site, following which 1 × 104 CD133+ cells were injected locally into the injured site. The magnetic force enabled the injected CD133+ cells to accumulate in the muscle injury site, with these cells also inhibiting fibrous scar formation and enhancing angiogenesis and myogenesis. Muscle injury was repaired by transplantation of CD133+ cells, which indicated that CD133+ cells are a suitable cell source for muscle repair, and our magnetic cell targeting system could promote the repair of muscle injury by transplantation of a limited number of CD133+ cells.
MiR-1, miR-133, and miR-206 are well known as potent regulators of muscle development and homeostasis. Because these miRNA are down regulated after muscle injury, then gradually increased thereafter, we investigated whether augmentation of miR-1, miR-133, and miR-203 by local injection could accelerate muscle regeneration in the rat tibialis anterior muscle laceration model [32]. One week post-injury, local injection of these miRNA accelerated muscle regeneration morphologically and physiologically. Fibrosis was effectively prevented and blood vessels increased in regenerated muscle. Local injection of miR-1, miR-133, and miR-206 could enhance muscle regeneration without use of cell implantation.
Nerves
In the field of cell therapy, cultured Schwann cells and neural progenitor cells are candidates for nerve regeneration, but have not yet been applied clinically because of technical and ethical problems. We hypothesized that it is feasible to apply CD133+ to peripheral nerve regeneration. A 15-mm defect in the sciatic nerve of athymic rats was bridged by use of a silicone tube with CD133+ cells embedded in atelocollagen gel [33]. Eight weeks after transplantation, the defect of the sciatic nerve was structurally regenerated by transplantation of CD133+ cells. The number of myelinated fibers, axon diameter, and myelin thickness were increased, and compound muscle action potentials were observed after CD133+ cell transplantation. Moreover, transplanted CD133+ cells differentiated into Schwann cells. Although transplantation of CD133+ cells could enhance recovery after peripheral nerve injury, the number of CD133+ cells obtained from peripheral blood is extremely limited. To address this problem, we transplanted ex-vivo-expanded CD133+ cells into a defect site in the rat sciatic nerve. The fresh CD133+ cells were cultured in serum-free medium supplemented with 100 ng/ml stem cell factor, 50 ng/ml interleukin-6, 20 ng/ml thrombopoietin, and 100 ng/ml FMS-like tyrosine kinase-3 ligand for 7 days [34]. After 1 week of expansion culture, the number of cells increased 9.6 ± 3.3-fold; expanded cells comprised 59.0 % CD133+ cells and 32.2 % CD133+/CD34+ cells, whereas fresh CD133+ cells comprised 93.2 % CD133+ cells and 91.2 % CD133+/CD34+ cells, on the basis of FACS analysis. The histological and functional results of an in-vivo study confirmed that regenerated nerve fibers in the defect were similar to the freshly transplanted CD133+ cells [35]. The transplantation of expanded CD133+ cells had the potential for nerve regeneration, which suggested that expanded CD133+ cell transplantation could develop into a novel therapeutic strategy for nerve regeneration.
Spinal cord
As a novel approach for spinal cord regeneration, we focused on the close interaction between the nervous system and blood vessels described as the “vascular niche”. CD133+ cells were administered intravenously in the rat spinal cord injury (SCI) model [36]. Transplantation of CD133+ cells could enhance intrinsic angiogenesis and axonal regeneration with significant functional recovery. In the mechanism of spinal cord regeneration, we proved that Jag1-Notch signaling from EPC contributes to promoting repair of the injured spinal cord by enhancement of astrogliosis [37]. With the purpose of increasing the limited number of CD133+ cells, ex-vivo-expanded CD133+ cells were transplanted intravenously into SCI mice [38]. Transplantation of fresh CD133+ and expanded cells similarly promoted improvement of spinal cord function after injury compared with administration of PBS or CD133− mononuclear cell (MNC). To establish a safe and secure cell transplantation method for SCI, a magnetic cell-delivery system was used. CD133+ cells were transplanted by the intrathecal route by use of a lumbar puncture in thoracic spinal cord injury models of immunodeficient rats, both in the presence of an external magnetic field and in the absence of an external magnetic field [39]. In-vivo fluorescence imaging showed that administered CD133+ cells accumulated at the injury site at the thoracic level in the presence of a magnetic field but that almost all administered CD133+ cells remained in the punctured area of lumber level in the absence of a magnetic field. Transplantation of CD133+ cells in the presence of a magnetic field promoted axon growth and functional recovery after spinal cord injury.
Future perspectives
iPSC will lead to a breakthrough in regenerative medicine, although several issues for clinical use remain to be solved. Because MSC and EPC have been already applied clinically, we believe our studies using these cells and materials will enable further development of clinical use in the near future. To obtain plenty of CD34+ or CD133+ cells, mobilization from bone marrow (in-vivo expansion) with G-CSF (granulocyte-colony-stimulating factor) and subsequent aphaeresis will be required, because of their low frequency. Therefore, as a future option, ex-vivo expansion of CD34+ or CD133+ cells by use of optimized medium containing growth factor/cytokine cocktails has been tried, to establish a less invasive and less costly approach for EPC-assisted tissue regeneration. This new therapeutic approach will enable more promising and comprehensive tissue repair/regeneration in orthopaedics. The immediate purpose is more effective and less invasive cell therapy, with spatial control of transplanted cells by use of an external magnetic force. Although there have been many reports of the effectiveness of cell therapy in tissue regeneration, its mechanism is yet to be fully elucidated. Analysis of miRNA/exosome in cell therapy has the potential to clarify this mechanism, thus leading to novel alternative cell therapy for tissue regeneration (Fig. 3). Several reports have revealed that therapeutic trials by administration of synthetic miRNA or modified antisense in vivo have been conducted in many fields, hence, miRNA therapy in clinical orthopaedics will be introduced in the near future. It is likely that the combination of miRNA and an external magnetic device will be more useful and safe, so any accompanying challenges should be examined to discover the most effective and least invasive methods.
The new therapeutic strategy of magnetically labeled MSC with external magnetic force for cartilage injury was adopted by the Japan Science and Technology Agency in Japan as the “Highway Program for Realization of Regenerative Medicine”, and we are now at the stage of realizing clinical application of cell therapy combined with a magnetic targeting system for cartilage and bone injury, after government approval. In conclusion, comprehensive analysis of the efficiency, safety, and mechanism of tissue regeneration for clinical application will lead to more feasible and promising regenerative medicine as novel next-generation therapy.
References
Lidgren L. The bone and joint decade 2000–2010: an update. Acta Orthop Scand. 2000;71(1):3–6.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76.
Ochi M. Shinya Yamanaka’s 2012 nobel prize and the radical change in orthopedic strategy thanks to his discovery of iPS cells. Acta Orthop. 2013;84(1):1–3.
Caplan AI. Mesenchymal stem cells. J Orhop Res. 1991;9(5):641–50.
Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–4.
Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–93.
Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4(3):214–22.
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–7.
Kobayashi T, Ochi M, Yanada S, Ishikawa M, Adachi N, Deie M, Arihiro K. A novel cell delivery system using magnetically labeled mesenchymal stem cells and an external magnetic device for clinical cartilage repair. Arthoscopy. 2008;24(1):69–76.
Kamei G, Kobayashi T, Ohkawa S, Kongcharoensombat W, Adachi N, Takazawa K, Shibuya H, Deie M, Hattori K, Goldberg JL, Ochi M. Articular cartilage repair with magnetic mesenchymal stem cells. Am J Sports Med. 2013;41(6):1255–64.
Ambros V. The functions of animal microRNA. Nature. 2004;431(7006):350–5.
Bartel DP. MicroRNA: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97.
Murata K, Yoshitomi H, Tanida S, Ishikawa M, Nishitani K, Ito H, Nakamura T. Plasma and synovial fluid microRNA as potential biomarkers of rheumatoid arthritis and osteoarthritis. Arthritis Res Ther. 2010;12(3):R86.
Uchio Y, Ochi M, Matsusaki M, Kurioka H, Katsube K. Human chondrocyte proliferation and matrix synthesis cultured in atelocollagen gel. J Biomed Mater Res A. 2000;50(2):138–43.
Minakuchi Y, Takeshita F, Kosaka N, Sasaki H, Yamamoto Y, Kouno M, Honma K, Nagahara S, Hanai S, Sano A, Kato T, Terada M, Ochiya T. Atelocollagen-mediated synthetic small interfering RNA delivery for effective gene silencing in vitro and in vivo. Nucleic Acids Res. 2004;32(13):e109.
Tamai N, Myoui A, Tomita T, Nakase T, Tanaka J, Ochi T, Yosikawa H. Novel hydroxyapatite ceramics with an interconnective porus structure exhibit superior osteoconduction in vivo. J Biomed Mater Res. 2001;59(1):110–7.
Kawasaki K, Ochi M, Uchio Y, Adachi N, Matsusaki M. Hyaluronic acid enhances proliferation and chondroitin sulfate synthesis in cultured chondrocytes embedded in collagen gels. J Cell Physiol. 1999;179(2):142–8.
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Eng J Med. 1994;331(14):889–95.
Ochi M, Uchio Y, Kawasaki K, Wakitani S, Iwasa J. Transplantation of cartilage-like tissue made by tissue engineering in the treatment of cartilage defects of the knee. J Bone Joint Surg Br. 2002;84(4):571–8.
Ito Y, Ochi M, Adachi N, Sugawara K, Yanada S, Ikada Y, Ronakorn P. Repair of osteochondral defect with tissue-engineered chondral plug in a rabbit model. Arthroscopy. 2005;21(10):1155–63.
Nishimori M, Deie M, Kanaya A, Exham H, Adachi N, Ochi M. Repair of chronic osteochondral defects in the rat. J Bone Joint Surg Br. 2006;88(9):1236–44.
Agung M, Ochi M, Yanada S, Adachi N, Izuta Y, Yamasaki T, Toda K. Mobilization of bone marrow-derived mesenchymal stem cells into the injured tissues after intraarticular injection and their contribution to tissue regeneration. Knee Surg Sports Traumatol Arthrosc. 2006;14(12):1307–14.
Ito Y, Tanaka N, Fujimoto Y, Yasunaga Y, Ishida O, Agung M, Ochi M. Bone formation using interconnected porous calcium hydroxyapatite ceramic hybridized with cultured marrow stromal stem cells derived from Green rat. J Biomed Mater Res. 2004;69A(3):454–61.
Oshima S, Ishikawa M, Mochizuki Y, Kobayashi T, Yasunaga Y, Ochi M. Enhancement of bone formation in an experimental bony defect using ferumoxide-labelled mesenchymal stromal cells and a magnetic targeting system. J Bone Joint Surg Br. 2010;92(11):1606–13.
Kodama A, Kamei G, Kongcharoensombat W, Ohkawa S, Nakabayashi A, Ochi M. In vivo bioluminescence imaging of transplanted bone marrow mesenchymal stromal cells using a magnetic delivery system in a rat fracture model. J Bone Joint Surg Br. 2012;94(7):998–1006.
Nagineni CN, Amiel D, Green MH, Berchuck M, Akeson WH. Characterization of the intrinsic properties of the anterior cruciate and medial collateral ligament cells. J Orthop Res. 1992;10(4):465–75.
Kanaya A, Deie M, Adachi N, Nishimori M, Yanada S, Ochi M. Intra-articular injection of mesenchymal stromal cells in partially torn anterior cruciate ligaments in a rat model. Arthroscopy. 2007;23(6):610–7.
Shoji T, Nakasa T, Yamasaki K, Kodama A, Miyaki S, Niimoto T, Ohuhara A, Kamei N, Adachi N, Ochi M. The effect of intra-articular injection of microRNA-210 on ligament healing in a rat model. Am J Sports Med. 2012;40(11):2470–8.
Natsu K, Ochi M, Mochizuki Y, Hachisuka H, Yanada S, Yasunaga Y. Allogenic bone marrow-derived mesenchymal stromal cells promote the regeneration of injured skeletal muscle without differentiation into myofibers. Tissue Eng. 2004;10(7–8):1093–112.
Shi M, Ishikawa M, Kamei N, Nakasa T, Adachi N, Deie M, Asahara T, Ochi M. Acceleration of skeletal muscle regeneration in a rat skeletal muscle injury model by local injection of human peripheral blood-derived CD133-positive cells. Stem Cells. 2009;27(4):949–60.
Ohkawa S, Kamei N, Kamei G, Shi M, Adachi N, Deie M, Ochi M. Magnetic targeting of human peripheral blood CD133+ cells for skeletal muscle regeneration. Tissue Eng Part C Methods. 2013;19(8):631–41.
Nakasa T, Ishikawa M, Shi M, Shibuya H, Adachi N, Ochi M. Acceleration of muscle regeneration by local injection of muscle-specific microRNA in rat skeletal muscle injury model. J Cell Mol Med. 2010;14(10):2495–505.
Kijima Y, Ishikawa M, Sunagawa T, Nakanishi K, Kamei N, Yamada K, Tanaka N, Kawamata S, Asahara T, Ochi M. Regeneration of peripheral nerve after transplantation of CD133+ cells derived from human peripheral blood. J Neurosurg. 2009;110(4):758–67.
Masuda H, Iwasaki H, Kawamoto A, Akimaru H, Ishikawa M, Ii M, Shizuno T, Sato A, Ito R, Horii M, Ishida H, Kato S, Asahara T. Development of serum-free quality and quantity control culture of colony forming endothelial progenitor cell expansion for vasculogenesis. Stem Cells Trans Med. 2012;1(2):160–71.
Ohtsubo S, Ishikawa M, Kamei N, Kijima Y, Suzuki O, Sunagawa T, Higashi Y, Masuda H, Asahara T, Ochi M. The therapeutic potential of ex vivo expanded CD133+ cells derived from human peripheral blood for peripheral nerve injuries. J Neurosurg. 2012;117(4):787–94.
Sasaki H, Ishikawa M, Tanaka N, Nakanishi K, Kamei N, Asahara T, Ochi M. Administration of human peripheral blood-derived CD133+ cells accelerates functional recovery in a rat spinal cord injury model. Spine. 2009;34(3):249–54.
Kamei N, Kwon SM, Ishikawa M, Ii M, Nakanishi K, Yamada K, Hozumi K, Kawamoto A, Ochi M, Asahara T. Endothelial progenitor cells promote astrogliosis following spinal cord injury through Jagged1-dependent Notch signaling. J Neurotrauma. 2012;29(9):1758–69.
Kamei N, Kwon SM, Alev C, Nakanishi K, Yamada K, Masuda H, Ishikawa M, Kawamoto A, Ochi M, Asahara T. Ex-vivo expanded human blood-derived CD133+ cells promote repair of injured spinal cord. J Neurol Sci. 2013;328(1–2):41–50.
Fujioka Y, Tanaka N, Nakanishi K, Kamei N, Nakamae T, Izumi B, Ohta R, Ochi M. Magnetic field-based delivery of human CD133+ cells promotes functional recovery after rat spinal cord injury. Spine. 2012;37(13):E768–77.
Acknowledgments
Our research described in this article was supported by MEXT KAKENHI Grant-in-Aid for Scientific Research (A) grant nos 21249079 and 25253089 and by the “Highway Program for Realization of Regenerative Medicine” from the Japan Science and Technology Agency. The contents of this article were presented at the 28th Annual Research Meeting of the Japanese Orthopaedic Association, October17–18, 2013.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Ochi, M., Nakasa, T., Kamei, G. et al. Regenerative medicine in orthopedics using cells, scaffold, and microRNA. J Orthop Sci 19, 521–528 (2014). https://doi.org/10.1007/s00776-014-0575-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00776-014-0575-6