Abstract
Background
Articular cartilage has a limited capacity for spontaneous repair, and its repair remains a clinical challenge. The purpose of this study was to prepare scaffold-free cartilage-like constructs and evaluate the feasibility of their use for the treatment of cartilage and osteocartilage defects in vivo.
Methods
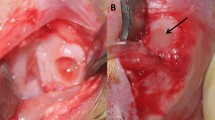
The scaffold-free constructs were prepared by chondrocytes isolated from the articular cartilage of rabbits using a high-density three-dimensional culture system. Two different defects, i.e., a chondral defect without oozing blood and an osteochondral defect with oozing blood, of 4-mm diameter, were created on the patellar groove of rabbits and forwarded to in vivo trials. In each defect, the constructs cut into 4-mm-diameter cylinders were grafted at the bottom of the defects. As a control, defects were only made on the contralateral knee joint in each rabbit. At 2, 4, 8 and 12 weeks after surgery, six rabbits in each group were evaluated macroscopically and histologically.
Results
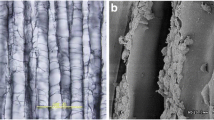
In vitro, histological examination revealed that the constructs have the character of hyaline cartilage with a potential adhesiveness to surrounding tissue. In vivo, in two control groups, incomplete spontaneous cartilage repair was observed in the osteochondral defects, whereas no repair was observed in the chondral defects. In the two treated groups, the surviving constructs in chondral defects showed significantly better repair compared to those in osteochondral defects.
Conclusions
It is possible for a chondral defect to be repaired by scaffold-free constructs in certain conditions. Establishing the optimal environment suitable for cartilage repair is warranted.








Similar content being viewed by others
References
Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–24.
Buschmann MD, Gluzband YA, Grodzinsky AJ, Kimura JH, Hunziker EB. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J Orthop Res. 1992;10:745–58.
Takahashi T, Ogasawara T, Asawa Y, Mori Y, Uchinuma E, Takato T, Hoshi K. Three-dimensional microenvironments retain chondrocyte phenotypes during proliferation culture. Tissue Eng. 2007;13:1583–92.
Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100.
Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol. 2008;20:109–16.
Mainil-Varlet P, Rieser F, Grogan S, Mueller W, Saager C, Jakob RP. Articular cartilage repair using a tissue-engineered cartilage-like implant: an animal study. Osteoarthr Cartil. 2001;9 Suppl A:S6–15.
Uenaka K, Imai S, Ando K, Matsusue Y. Relation of low-intensity pulsed ultrasound to the cell density of scaffold-free cartilage in a high-density static semi-open culture system. J Orthop Sci. 2010;15:816–24.
Freed LE, Martin I, Vunjak-Novakovic G. Frontiers in tissue engineering. In vitro modulation of chondrogenesis. Clin Orthop Relat Res. 1999;367 Suppl:S46–58.
Kumagai K, Saito T, Koshino T. Articular cartilage repair of rabbit chondral defect: promoted by creation of periarticular bony defect. J Orthop Sci. 2003;8:700–6.
Schaefer D, Martin I, Jundt G, Seidel J, Heberer M, Grodzinsky A, Bergin I, Vunjak-Novakovic G, Freed LE. Tissue-engineered composites for the repair of large osteochondral defects. Arthr Rheum. 2002;46:2524–34.
Wei X, Gao J, Messner K. Maturation-dependent repair of untreated osteochondral defects in the rabbit knee joint. J Biomed Mater Res. 1997;34:63–72.
Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, Goldberg VM. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76:579–92.
Matsuda H, Kitamura N, Kurokawa T, Arakaki K, Gong JP, Kanaya F, Yasuda K. Influence of the gel thickness on in vivo hyaline cartilage regeneration induced by double-network gel implanted at the bottom of a large osteochondral defect: short-term results. BMC Musculoskelet Disord. 2013;14:50.
Mankin HJ. Localization of tritiated thymidine in articular cartilage of rabbits II. Repair in immature cartilage. J Bone Joint Surg. 1962;44:688–98.
Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75:532–53.
Woo SL, Buckwalter JA. AAOS/NIH/ORS workshop. Injury and repair of the musculoskeletal soft tissues. Savannah, Georgia, June 18–20, 1987. J Orthop Res. 1988;6:907–31.
Lin L, Zhou C, Wei X, Hou Y, Zhao L, Fu X, Zhang J, Yu C. Articular cartilage repair using dedifferentiated articular chondrocytes and bone morphogenetic protein 4 in a rabbit model of articular cartilage defects. Arthr Rheum. 2008;58:1067–75.
Boopalan PR, Arumugam S, Livingston A, Mohanty M, Chittaranjan S. Pulsed electromagnetic field therapy results in healing of full thickness articular cartilage defect. Int Orthop. 2011;35:143–8.
Wang W, Li B, Li Y, Jiang Y, Ouyang H, Gao C. In vivo restoration of full-thickness cartilage defects by poly(lactide-co-glycolide) sponges filled with fibrin gel, bone marrow mesenchymal stem cells and DNA complexes. Biomaterials. 2010;31:5953–65.
Okano T, Yamada N, Sakai H, Sakurai Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide). J Biomed Mater Res. 1993;27:1243–51.
Buckwalter JA. Articular cartilage: injuries and potential for healing. J Orthop Sports Phys Ther. 1998;28:192–202.
Chen J, Maniwa S, Ochi M. Influence of trypsin on the biological bonding of cartilaginous surface to bone in rabbits. Arch Orthop Trauma Surg. 2000;120:587–91.
Brehm W, Aklin B, Yamashita T, Rieser F, Trüb T, Jakob RP, Mainil-Varlet P. Repair of superficial osteochondral defects with an autologous scaffold-free cartilage construct in a caprine model: implantation method and short-term results. Osteoarthr Cartil. 2006;14:1214–26.
Marlovits S, Striessnig G, Kutscha-Lissberg F, Resinger C, Aldrian SM, Vécsei V, Trattnig S. Early postoperative adherence of matrix-induced autologous chondrocyte implantation for the treatment of full-thickness cartilage defects of the femoral condyle. Knee Surg Sports Traumatol Arthrosc. 2005;13:451–7.
Ho ST, Hutmacher DW, Ekaputra AK, Hitendra D, Hui JH. The evaluation of a biphasic osteochondral implant coupled with an electrospun membrane in a large animal model. Tissue Eng Part. 2010;16:1123–41.
Schreiber RE, Ilten-Kirby BM, Dunkelman NS, Symons KT, Rekettye LM, Willoughby J, Ratcliffe A. Repair of osteochondral defects with allogeneic tissue engineered cartilage implants. Clin Orthop Relat Res. 1999;367 Suppl:S382–95.
Acknowledgments
We gratefully acknowledge Yoko Uratani for her technical assistance and thank Takefumi Yamamoto (Central Research Laboratory, Shiga University of Medical Science) for technical support regarding histological examinations. We also thank Drs. Mitsuhiko Kubo and Fumiyoshi Kojima for their advice.
Conflict of interest
We did not have any financial or personal relationships with other people or organizations that could inappropriately influence (bias) our work. Neither we nor a member of our immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity. No commercial entity paid or directed, or agreed to pay or direct, any benefits to any research fund, foundation, division, center, clinical practice, or other charitable or nonprofit organization related to the authors or a member of their immediate families, are affiliated or associated.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Oda, K., Mori, K., Imai, S. et al. Comparison of repair between cartilage and osteocartilage defects in rabbits using similarly manipulated scaffold-free cartilage-like constructs. J Orthop Sci 19, 637–645 (2014). https://doi.org/10.1007/s00776-014-0574-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00776-014-0574-7