Abstract
Background
During the process of degenerative aging of the intervertebral disc (IVD), the extracellular matrix (ECM) environment changes, with osmolarity and oxygen (O2) concentration important components of such changes. The IVD cells respond to maintain the homeostasis and function of the IVD by several mechanisms. Aquaporin-1 (AQP-1) is a transmembrane channel protein that is permeable to water and O2, which prevents rapid volume deformation under osmotic stress and facilitates O2 diffusion across the plasma membrane. One hypothesis is that AQP-1 has potential roles in aging degeneration of IVDs.
Methods
In this study, AQP-1 expression levels were investigated in aging rabbit nucleus pulposus (NP) cells using immunohistochemistry and Western blotting in vivo, and different osmolarities and O2 concentrations in vitro by quantitative real-time PCR.
Results
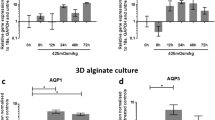
The results showed that AQP-1 was expressed at different levels in aging rabbit’s NPs and AQP-1 was regulated by the NP cells in different ECM environmental conditions. AQP-1 was downregulated under hypo-osmotic stress to prevent rapid swelling deformation and was upregulated under hypoxic stress to facilitate O2 utilization.
Conclusion
It is suggested that AQP-1 may reflect the status of aged IVDs and have a potential role in reflecting the adaptability of NP cells under different adverse ECM environments in aging degenerated IVDs.




Similar content being viewed by others
References
Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine. 2004;29:2700–9.
Urban JP, Marouda A. The measurement of fixed charge density in the intervertebral disc. Biochim Biophys Acta. 1979;586:166–78.
Tran ND, Kim S, Vincent HK, Rodriguez A, Hinton DR, Bullock MR, Young HF. Aquaporin-1-mediated cerebral edema following traumatic brain injury: effects of acidosis and corticosteroid administration. J Neurosurg. 2010;112:1095–104.
Nesic O, Lee J, Unabia GC, Johnson K, Ye Z, Vergara L, Hulsebosch CE, Perez-Polo JR. Aquaporin 1—a novel player in spinal cord injury. J Neurochem. 2008;105:628–40.
Xiong X, Miao J, Xi Z, Zhang H, Han B, Hu Y. Regulatory effect of dexamethasone on aquaporin-1 expression in cultured bovine trabecular meshwork cells. J Huazhong Univ Sci Technol Med Sci. 2005;25:735–7.
Sonoda H, Yokota-Ikeda N, Oshikawa S, Kanno Y, Yoshinaga K, Uchida K, Ueda Y, Kimiya K, Uezono S, Ueda A, Ito K, Ikeda M. Decreased abundance of urinary exosomal aquaporin-1 in renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2009;297:F1006–16.
Trujillo E, González T, Marín R, Martín-Vasallo P, Marples D, Mobasheri A. Human articular chondrocytes, synoviocytes and synovial microvessels express aquaporin water channels; upregulation of AQP1 in rheumatoid arthritis. Histol Histopathol. 2004;19:435–44.
Mobasheri A, Moskaluk CA, Marples D, Shakibaei M. Expression of aquaporin 1 (AQP1) in human synovitis. Ann Anat. 2010;192:116–21.
Mobasheri A, Marples D. Expression of the AQP-1 water channel in normal human tissues: a semiquantitative study using tissue microarray technology. Am J Physiol Cell Physiol. 2004;286:C529–37.
Richardson SM, Knowles R, Marples D, Hoyland JA, Mobasheri A. Aquaporin expression in the human intervertebral disc. J Mol Histol. 2008;39:303–9.
Leung VY, Hung SC, Li LC, Wu EX, Luk KD, Chan D, Cheung KM. Age-related degeneration of lumbar intervertebral discs in rabbits revealed by deuterium oxide-assisted MRI. Osteoarthr Cartil. 2008;16:1312–8.
Sowa G, Vadalà G, Studer R, Kompel J, Iucu C, Georgescu H, Gilbertson L, Kang J. Characterization of intervertebral disc aging: longitudinal analysis of a rabbit model by magnetic resonance imaging, histology, and gene expression. Spine. 2008;33:1821–8.
Gan JC, Ducheyne P, Vresilovic EJ, Shapiro IM. Intervertebral disc tissue engineering II: cultures of nucleus pulposus cells. Clin Orthop Relat Res. 2003;411:315–24.
Bibby SR, Urban JP. Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur Spine J. 2004;13:695–701.
Boyd LM, Richardson WJ, Chen J, Kraus VB, Tewari A, Setton LA. Osmolarity regulates gene expression in intervertebral disc cells determined by gene array and real-time quantitative RT-PCR. Ann Biomed Eng. 2005;33:1071–7.
Xia M, Zhu Y. Expression of integrin subunits in the herniated intervertebral disc. Connect Tissue Res. 2008;49:464–9.
Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in Basic Science. Spine. 2002;27:2631–44.
Brown MD, Tsaltas T. Studies on the permeability of the intervertebral disc during skeletal maturation. Spine. 1976;1:120–4.
Echevarría M, Muñoz-Cabello AM, Sánchez-Silva R, Toledo-Aral JJ, López-Barneo J. Development of cytosolic hypoxia and hypoxia-inducible factor stabilization are facilitated by aquaporin-1 expression. J Biol Chem. 2007;282:30207–15.
Maroudas A. Biophysical chemistry of cartilaginous tissues with special reference to solute and fluid transport. Biorheology. 1975;12:233–48.
Holm S, Maroudas A, Urban JP, Selstam G, Nachemson A. Nutrition of the intervertebral disc: solute transport and metabolism. Connect Tissue Res. 1981;8:101–19.
Takeno K, Kobayashi S, Negoro K, Uchida K, Miyazaki T, Yayama T, Shimada S, Baba H. Physical limitations to tissue engineering of intervertebral disc cells: effect of extracellular osmotic change on glycosaminoglycan production and cell metabolism. Laboratory investigation. J Neurosurg Spine. 2007;7:637–44.
Ishihara H, Warensjo K, Roberts S, Urban JP. Proteoglycan synthesis in the intervertebral disk nucleus: the role of extracellular osmolality. Am J Physiol. 1997;272:C1499–506.
Pritchard S, Erickson GR, Guilak F. Hyperosmotically induced volume change and calcium signaling in intervertebral disk cells: the role of the actin cytoskeleton. Biophys J. 2002;83:2502–10.
Ishihara H, Urban JP. Effects of low oxygen concentrations and metabolic inhibitors on proteoglycan and protein synthesis rates in the intervertebral disc. J Orthop Res. 1999;17:829–35.
Okada Y, Maeno E, Shimizu T, Dezaki K, Wang J, Morishima S. Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD). J Physiol. 2001;532:3–16.
Rouzaire-Dubois B, Ouanounou G, O’Regan S, Dubois JM. Sodium-dependent activity of aquaporin-1 in rat glioma cells: a new mechanism of cell volume regulation. Pflügers Archiv. Eur J Physiol. 2009;457:1187–98.
Fischbarg J, Diecke FP, Iserovich P, Rubashkin A. The role of the tight junction in paracellular fluid transport across corneal endothelium. Electro-osmosis as a driving force. J Membr Biol. 2006;210:117–30.
Tsai TT, Guttapalli A, Agrawal A, Albert TJ, Shapiro IM, Risbud MV. MEK/ERK signaling controls osmoregulation of nucleus pulposus cells of the intervertebral disc by transactivation of TonEBP/OREBP. J Bone Miner Res. 2007;22:965–74.
Jiang Q, Cao C, Lu S, Kivlin R, Wallin B, Chu W, Bi Z, Wang X, Wan Y. MEK/ERK pathway mediates UVB-induced AQP1 downregulation and water permeability impairment in human retinal pigment epithelial cells. Int J Mol Med. 2009;23:771–7.
Ohshima H, Urban JP. The effect of lactate and pH on proteoglycan and protein synthesis rates in the intervertebral disc. Spine. 1992;17:1079–82.
Conflict of interest
We report and confirm that there are no conflicts of interest. We alone are responsible for the content and writing of our paper.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Wang, F., Zhu, Y. Aquaporin-1: a potential membrane channel for facilitating the adaptability of rabbit nucleus pulposus cells to an extracellular matrix environment. J Orthop Sci 16, 304–312 (2011). https://doi.org/10.1007/s00776-011-0055-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00776-011-0055-1