Abstract
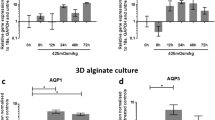
Intervertebral disc (IVD) cells experience a broad range of physicochemical stimuli under physiologic conditions, including alterations in their osmotic environment. Cellular responses to altered osmolarity have been documented at the transcriptional and post-translational level, but mainly for extracellular matrix proteins. In this study, the gene expression profile of human IVD cells was quantified with gene array technology following exposure to increased osmolarity in order to capture the biological responses for a broad set of targets. A total of 42 genes were identified in IVD cells as significantly changed following culture under hyper-osmotic conditions. Gene expression patterns were verified using RT-PCR. Genes identified in this study include those related to cytoskeleton remodeling and stabilization (ephrin-B2, muskelin), as well as membrane transport (ion transporter SLC21A12, osmolyte transporter SLC5A3, monocarboxylic acid SLC16A6). An unexpected finding was the differential regulation of the gene for the neurotrophin, brain-derived neurotrophic factor, by hyper-osmotic stimuli that suggests a capability of IVD cells to respond to physicochemical stimuli with factors that may regulate discogenic pain.
Similar content being viewed by others
References
Apfel, S. C. Neurotrophic factors and pain. Clin. J. Pain 16:S7–S11, 2000.
Baer, A. E., J. Y. Wang, V. B. Kraus, and L. A. Setton. Collagen gene expression and mechanical properties of intervertebral disc cell-alginate cultures. J. Orthop. Res. 19:2–10, 2001.
Bakay, M., Y.-W. Chen, R. Borup, P. Zhao, K. Nagaraju, and E. P. Hoffman. Sources of variability and effect of experimental approach on expression profiling data interpretation. BMC Bioinform. 3:2002.
Battaglia, A. A., K. Sehayek, J. Grist, S. B. McMahon, and I. Gavazzi. Eph-b receptors and ephrin-b ligands regulate spinal sensory connectivity and modulate pain processing. Nat. Neurosci 6:339–340, 2003.
Bayliss, M. T., B. Johnstone, and J. P. O’Brien. Proteoglycan synthesis in the human intervertebral disc. Variation with age, region and pathology. Spine 13:972–981, 1988.
Bayliss, M. T., J. P. Urban, B. Johnstone, and S. Holm. In vitro method for measuring synthesis rates in intervertebral disc. J. Orthop. Res. 4:10–17, 1986.
Bennett, D. L. H. Neurotrophic factors: Important regulators of nociceptive function. Neurosci. Update 7:13–17, 2001.
Bush, P. G., and A. C. Hall. The osmotic sensitivity of isolated and in situ bovine articular chondrocytes. J. Orthop. Res. 19:768–778, 2001.
Caterson, B., C. R. Flannery, C. E. Hughes, and C. B. Little. Mechanisms involved in cartilage proteoglycan catabolism. Matrix Biol. 19:333–244, 2000.
Chen, J., A. E. Baer, P. Y. Paik, W. Yan, and L. A. Setton. Matrix protein gene expression in intervertebral disc cells subjected to altered osmolarity. Biochem. Biophys. Res. Commun. 293:932–938, 2002.
Chiba, K., G. B. J. Andersson, K. Masuda, and E. J.-M. A. Thonar. Metabolism of the extracellular matrix formed by intervertebral disc cells cultured in alginate. Spine 22:2885–2893, 1997.
Cutforth, T., and C. J. Harrison. Ephs and ephrins close ranks. Trends Neurosci. 25:332–334, 2002.
Dodelet, V. C., and E. B. Pasquale. Eph receptors and ephrin ligands: Embryogenesis to tumorigenesis. Oncogene 19:5614–5619, 2000.
Erickson, G. R., L. G. Alexopoulos, and F. Guilak. Hyper-osmotic stress induces volume change and calcium transients in chondrocytes by transmembrane, phospholipid, and G-protein pathways. J. Biomech. 34:1527–1535, 2001.
Gradient, R. A., and U. H. Otten. Interleukin-6 (IL-6) - a molecule with both beneficial and destructive potentials. Prog. Neurobiol. 52:379–390, 1997.
Ha, S. O., J. K. Kim, H. S. Hong, D. S. Kim, and H. J. Cho. Expression of brain-derived neurotrophic factor in rat dorsal root ganglia, spinal cord and gracile nuclei in experimental models of neuropathic pain. Neuroscience 107:301–309, 2001.
Han, E.-S., Y. Wu, R. McCarter, J. F. Nelson, A. Richardson, and S. G. Hilsenbeck. Reproducibility, sources of variability, pooling, and sample size: Important considerations for the design of high-density oligonucleotide array experiments. J. Gerontol. Biol. Sci. 59A:306–315, 2004.
Hirano, T., and T. Kishimoto. “Interleukin-6.” In: Peptide Growth Factors and Their Receptors, edited by Sporn, M. B. and A. B. Roberts. New York: Springer-Verlag, 1991, pp. 633–665.
Holder, N., and R. Klein. Eph receptors and ephrins: Effectors of morphogenesis. Development 126:2033–2044, 1999.
Hopewell, B., and J. P. G. Urban. Adaptation of articular chondrocytes to changes in osmolality. Biorheology 20:73–77, 2003.
Ishihara, H., K. Warensjo, S. Roberts and J. P. G. Urban. Proteoglycan synthesis in the intervertebral disk nucleus: The role of extracellular osmolality. Am. J. Physiol. 272:C1499–C1506, 1997.
Kuno, K., N. Kanada, E. Nakashima, F. Fujiki, F. Ichimura, and K. Matsushima. Molecular cloning of a gene encoding a new type of metalloproteinase-disintegrin family protein with thrombospondin motiffs as an inflammation associated gene. J. Biol. Chem. 272:556–562, 1997.
Lang, F., G. L. Busch, M. Ritter, H. Volkl, S. Waldegger, E. Gulbins, and D. Haussinger. Functional significance of cell volume regulatory mechanisms. Physiol. Rev. 78:247–306, 1998.
Livak, K. J., and T. D. Schmittgen. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods 25:402–408, 2001.
Maldonado, B. A., and J. Theodore R. Oegema. Initial characterization of the metabolism of intervertebral disc cells encapsulated in microspheres. J. Orthop. Res. 10:677–690, 1992.
Mannion, R. J., M. Costigan, I. Decosterd, F. Amaya, Q. P. Ma, J. C. Holstege, R. R. Ji, A. Acheson, R. M. Lindsay, A. A. Wilkinson, and C. J. Woolf. Neurotrophins: Peripherirally and centrally acting modulators of tactile stimulus-induced inflammatory pain hypersensitivity. Proc. Natl. Acad. Sci. U.S.A. 96:9385–9390, 1999.
Miletic, G., and V. Miletic. Increase in the concentration of brain derived neurotrophic factor in the lumbar spinal dorsal horn are associated with pain behavior following chronic constriction injury in rats. Neurosci. Lett. 319:137–140, 2002.
Millan, M. J. The induction of pain: An integrative review. Prog. Neurobiol. 57:1–164, 1999.
O’Neill, W. C. Physiological significance of volume-regulatory transporters. Am. J. Physiol. Cell Physiol. 45:C995–C1011, 1999.
Obata, K., H. Tsujino, H. Yamanaka, D. Yi, T. Fukuoka, N. Hashimoto, K. Yonenobu, H. Yoshikawa, and K. Noguchi. Expression of neurotrophic factors in the dorsal root ganglion in a rat model of lumbar disc herniation. Pain 99:121–132, 2002.
Oegema, T. R. Biochemistry of the intervertebral disc. Clin. Sports Med. 12:419–439, 1993.
Pritchard, S., G. R. Erickson, and F. Guilak. Hyperosmotically induced volume change and calcium signalling in intervertebral disc cells: The role of the actin cytoskeleton. Biophys. J. 83:2502–2510, 2002.
Pritchard, S., and F. Guilak. The role of F-actin in hypo-osmotically induced cell volume change and calcium signaling in anulus fibrosus cells. Ann. Biomed. Eng. 32:103–111, 2004.
Rajeevan, M. S., S. D. Vernon, N. Taysavang, and E. R. Unger. Validation of array-based gene expression profiles by real-time (kinetic) RT-PCR. J. Mol. Diagnostics 3:26–31, 2001.
Specchia, N., A. Pagnotta, A. Toesca, and F. Greco. Cytokines and growth factors in the protruded intervertebral disc of the lumbar spine. Eur. Spine J. 11:145–151, 2002.
Urban, J. P. G. “The effect of physical factors on disk cell metabolism.” In: Musculoskeletal Soft-Tissue Aging: Impact on Mobility, edited by Buckwalter, J. A., Goldberg, V. M., and Woo, S. L.-Y. Rosemont. Illinois: AAOS, 1993, pp. 391–412.
Urban, J. P. G. The role of the physicochemical environment in determining disc cell behavior. Biochem. Soc. Trans. 30:858–864, 2002.
Urban, J. P. G., S. Roberts, and J. R. Ralphs. The nucleus of the intervertebral disc from development to degeneration. Am. Zool. 40:53–61, 2000.
Waldegger, S., and F. Lang. Cell volume and gene expression. J. Membr. Biol. 162:95–100, 1998.
Wang, J. Y., A. E. Baer, V. B. Kraus, and L. A. Setton. Intervertebral disc cells exhibit differences in gene expression in alginate and monolayer culture. Spine 26:1747–1751, 2001.
Yuen, T., E. Wurmbach, R. L. Pfeffer, B. J. Ebersole, and S. C. Sealfon. Accuracy and calibration of commercial oligonucleotide and custom cdna microarrays. Nucl. Acids Res. 30:e48, 2002.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Boyd, L.M., Richardson, W.J., Chen, J. et al. Osmolarity Regulates Gene Expression in Intervertebral Disc Cells Determined by Gene Array and Real-Time Quantitative RT-PCR. Ann Biomed Eng 33, 1071–1077 (2005). https://doi.org/10.1007/s10439-005-5775-y
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10439-005-5775-y