Abstract
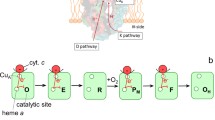
Electrons from cytochrome c, the substrate of cytochrome oxidase, a redox-linked proton pump, are accepted by CuA in subunit II. From there they are transferred to the proton pumping machinery in subunit I, cytochrome a and cytochrome a 3–CuB. The reduction of the latter site, which is the dioxygen reducing unit, is coupled to proton uptake. Dioxygen reduction involves a peroxide and a ferryl ion intermediate, and it is the transition between these and back to the resting oxidized enzyme that are coupled to proton pumping. The X-ray structures suggest electron–transfer pathways that can account for the observed rates provided that the reorganization energies are small. They also reveal two proton-transfer pathways, and mutagenesis experiments have shown that one is used for proton uptake during the initial reduction of cytochrome a 3–CuB, whereas the other mediates transfer of the pumped protons.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 23 March 1998 / Accepted: 11 May 1998
Rights and permissions
About this article
Cite this article
Malmström, B. Cytochrome oxidase: pathways for electron tunneling and proton transfer. JBIC 3, 339–343 (1998). https://doi.org/10.1007/s007750050242
Issue Date:
DOI: https://doi.org/10.1007/s007750050242