Abstract
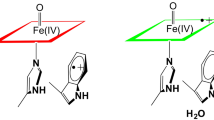
The heme enyzmes cytochrome c peroxidase (CCP) and pea cytosolic ascorbate peroxidase (APX) show a high level of sequence identity. The main difference near the active sites is the presence of a cation binding site in APX located about 1 nm from the Trp-179 side chain, which is hydrogen-bonded to Asp-208. It is possible that this difference in electrostatics provided by the protein environment is an essential determinant of the stabilization of the ion-pair or neutral form of the Trp...Asp couple in APX and CCP. Semiempirical molecular orbital calculations support the hypothesis that the position of the moving proton inside the couple influences the location of the free electron, leading to radical formation either on the heme or on the Trp side chain of these enzymes.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received, accepted: 26 November 1996
Rights and permissions
About this article
Cite this article
Náray-Szabó, G. Electrostatic modulation of electron transfer in the active site of heme peroxidases. JBIC 2, 135–138 (1997). https://doi.org/10.1007/s007750050117
Issue Date:
DOI: https://doi.org/10.1007/s007750050117