Abstract
Antimicrobial resistance (AMR) has been emerging as a major global health threat and calls for the development of novel drug candidates. Metal complexes have been demonstrating high efficiency as antibacterial agents that differ substantially from the established types of antibiotics in their chemical structures and their mechanism of action. One strategy to exploit this potential is the design of metal-based hybrid organometallics that consist of an established antibiotic and a metal-based warhead that contributes an additional mechanism of action different from that of the parent antibiotic. In this communication, we describe the organometallic hybrid antibiotic 2c, in which the drug metronidazole is connected to a gold(I) N-heterocyclic carbene warhead that inhibits bacterial thioredoxin reductase (TrxR). Metronidazole can be used for the treatment with the obligatory anaerobic pathogen Clostridioides difficile (C. difficile), however, resistance to the drug hampers its clinical success. The gold organometallic conjugate 2c was an efficient inhibitor of TrxR and it was inactive or showed only minor effects against eucaryotic cells and bacteria grown under aerobic conditions. In contrast, a strong antibacterial effect was observed against both metronidazole-sensitive and -resistant strains of C. difficile. This report presents a proof-of-concept that the design of metal-based hybrid antibiotics can be a viable approach to efficiently tackle AMR.
Graphical abstract
A metronidazole-gold hybrid metalloantibiotic with high efficacy against resistant C. difficile

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antimicrobial resistance (AMR) has become a global health problem with a sharply increasing relevance in the treatment of bacterial infections [1]. Metal-based drugs might play a key role in fighting AMR, as they differ significantly from the existing types of antibiotics and could in consequence be less susceptible to resistance of microbial pathogens. A systematic survey of the antibacterial activity of various types of metals implies their exceptional, yet hardly leveraged potential for antibiotic drug discovery [2,3,4].
Among the many investigated metal-based antibacterial agents, gold complexes have been attracting increasing attention recently [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21]. Gold complexes are among the strongest inhibitors of the enzyme thioredoxin reductase, which is involved in the cellular redox regulation of both eukaryotic and many prokaryotic cells. In particular many Gram-positive bacteria, which lack a glutathione/glutathione reductase system, are highly dependent on a functional TrxR. For the gold-containing therapeutic auranofin it has been convincingly demonstrated that this mechanism is connected to its antibacterial effects and the drug, approved for other indications, has been considered for repurposing as an antibacterial agent [6, 19, 22]. The inhibition of bacterial TrxR has been confirmed for an increasing number of different gold compounds, suggesting that the inhibition of this enzyme could be a key player for the antibacterial effects of gold compounds in general [8, 11, 20, 21, 23]. Because auranofin and many other gold complexes trigger cytotoxic effects in eukaryotic (i.e. human) cells, their potential as safe antibiotics might be limited.
Recently, we have reported on organometallic gold(I/III) complexes with N-heterocyclic carbene (NHC) ligands, many of which are efficient inhibitors of bacterial TrxR (see Fig. 1a for an example) [8, 20, 21, 24, 25]. Importantly, in agreement with the proposed mechanism of action based on TrxR inhibition, the complexes were highly active against Gram-positive bacteria, but poorly active or inactive against Gram-negative bacteria. However, the proliferation inhibition in human cancer and Gram-positive bacterial cells remained in a comparable concentration range.
Structures of the anti-infective gold NHC complex 1a [20] and metronidazole
One strategy to overcome toxicity limitations and AMR at the same time, is the design of metal-based hybrid antibiotics [26,27,28,29]. Such conjugates consist of an established antibacterial drug and a metal partial structure, which triggers antibacterial effects by a mode of action that is different from that of the selected antibiotic [2]. Here we selected metronidazole (Fig. 1) for the design of gold organometallic hybrid antibiotics, which is an antibiotic of the nitroimidazole class that inhibits nucleic acid synthesis by forming nitroso radicals. Metronidazole is activated by (partial) reduction that occurs usually in anaerobic bacteria, whereas the drug has little activity against aerobic bacteria and human cells. Metronidazole is, for example, applied in the therapy of infections with the obligate anaerobic bacterium Clostridioides difficile (C. difficile), which causes severe gastrointestinal infections. However, treatment success is diminished by the occurrence of metronidazole resistance. Based on the Gram-positive nature of C. difficile, we hypothesized that a TrxR-inhibiting gold organometallic conjugate with metronidazole could restore the activity against metronidazole-resistant C. difficile. In this report we provide the proof-of-concept for this approach.
Results
The hydroxy group of metronidazole offers an ideal position for forming conjugates without changing the pharmacophore. Regarding the gold NHC moiety, we selected complex 1a, for which we had observed antibacterial activity and TrxR inhibition recently [20], as a starting point for attaching metronidazole by a two-step ester hydrolysis/re-esterification at the benzimidazole backbone of the NHC (see scheme 1). Similarly, complex 2a was designed to introduce metronidazole at the side chain of the NHC ligand. Accordingly, the free carboxylic acids 1b [30] and 2b were obtained from 1 and 2a by reaction with NaOH. The target hybrid organometallics 1c and 2c were prepared by Steglich-like esterification with the hydroxy group of metronidazole. This coupling strategy had been previously successfully applied by Gibson et al. for other metal-based drug candidates, i.e. platinum(IV) complexes [31, 32].
The compounds were characterized by 1H- and 13C-NMR and mass spectroscopy, which were fully consistent with the proposed structures. The high purity of all compounds was confirmed by elemental analyses that differed less than 0.5% from the theoretical values.
The two conjugates 1c and 2c differ in their solubility in organic solvents. While 2c was soluble in most organic solvents and could be purified by column chromatography with dichloromethane and methanol as eluents, 1c showed limited solubility in organic solvents that prevented chromatographic purification. Such different properties might be the consequence of the more rigid nature of the ester moiety of 1c at the aromatic ring system, in comparison to the more flexible ester moiety of 2c.
The ability of the organometallic hybrid antibiotics 1c and 2c to inhibit bacterial TrxR was evaluated in an assay using the purified enzyme from E. coli in comparison to the gold complexes auranofin and the free acids 1b and 2b. In general, all gold complexes were very efficient inhibitors of TrxR with IC50 values in the range of 0.15–0.36 µM (Table 1). The most active inhibitors were the metronidazole conjugates 1c and 2c with IC50 values that were approximately half of those of the respective free acid derivatives, 1b and 2b, and slightly surpassed the activity of the reference drug auranofin.
Low cytotoxicity against mammalian cells is of advantage when developing novel antibiotics. Here, the antiproliferative effects were examined in human A-549 lung cancer, HT-29 colon carcinoma, MDA-MB-321 breast cancer and Vero E6 non-tumorigenic epithelial kidney cells of the African green monkey (Table 2). Metronidazole was inactive as expected (all IC50 values > 100 µM). The free acid derivatives, 1b and 2b, as well as the metronidazole conjugates, 1c and 2c, were also largely inactive (all IC50 values > 60 µM) except for minor antiproliferative effects of 2c in MDA-MB-231 cells (IC50 = 31.8 µM). Complex 2a, which is the ethyl ester derivative of 2c, was included in the study and showed minor to moderate cytotoxicity (IC50 values in the range of 9–26 µM, 9.6 µM against MDA-MB-231) that was in all cases 3–4 times stronger than that of the metronidazole conjugate 2c. Such a strong reduction of the antiproliferative effects upon coupling of the gold carbene moiety to metronidazole is highly promising regarding a possible safe application of the complexes as antibiotics.
The antibacterial effects were determined in a panel of aerobic Gram-positive (methicillin-resistant S. aureus MRSA, Enterococcus faecium) and Gram-negative (Escherichia coli, Pseudomonas aeruginosa, Acinetobacter baumannii, Klebsiella pneumoniae) pathogenic bacteria strains as minimal inhibitory concentration (MIC values) (Table 3). Metronidazole was not active under the aerobic conditions as expected. The gold reference compound auranofin showed strong activity against the Gram-positive stains and was lower active or inactive against the Gram-negative strains, in line with previous reports [6, 20, 25]. The free acid derivatives 1b and 2b were inactive (MIC values > 100 µM) against all bacteria strains. Except for 1c in MRSA, the metronidazole conjugates 1c and 2c showed only moderate activity (MIC values: 37–44 µM) against Gram-positive strains and were also inactive against all the Gram-negative bacteria. The preference for Gram-positive bacteria is in good agreement with our recent reports on various gold NHC complexes [6, 20, 25].
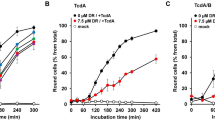
Next, we evaluated the antibacterial activity of 2c in concentrations up to 100 µM against two metronidazole-sensitive (1296 T, VPI10463) and one metronidazole-resistant IB136 (NCTC 14385)[33] strain of C. difficile in comparison to metronidazole under anaerobic conditions (Fig. 2). Preliminary studies with 1c had been hampered by solubility issues under the assays conditions and therefore it was excluded from further experiments. Metronidazole displayed the expected strong activity against the sensitive strains at concentrations of 12.5 µM and above, leading to a complete block of bacterial growth without recovery, whereas no dosage of the drug could terminate the proliferation of the resistant strain. Complex 2c blocked bacterial growth at the same concentration in all strains, including the metronidazole-resistant strain IB136 (NCTC14385), clearly confirming its ability to overcome metronidazole resistance in C. difficile. In addition, the activity against the highly toxin-producing strain VPI10463 is of particular interest, as toxins are causative for the pathogenicity of C. difficile. At a concentration of 6.25 µM both metronidazole and 2c were effective inhibitors of the sensitive bacteria, however, with a recovery of growth after extended exposure. At the same concentration against the resistant C. difficile strain IB136, 2c also showed strong effects with recovery of bacterial proliferation, whereas metronidazole remained completely inactive. To evaluate the importance of the metronidazole partial structure of 2c, complex 2b without metronidazole partial structure was evaluated under identical conditions and remained inactive against all C. difficile strains (Figures S1–S3), suggesting that the intact hybrid antibiotic is required for triggering bioactivity.
Conclusions
Conjugates 1c and 2c, which consist of a gold NHC structure linked to metronidazole, were prepared and characterized. The TrxR inhibitory activity of the gold NHC moiety was not only maintained in the two metronidazole conjugates, in fact 1c and 2c surpassed the activity of the respective free acid derivatives 1b and 2b. Both organometallic hybrid antibiotics 1c and 2c were non-toxic or showed low effects against human cancer cell lines, a mammalian normal cell line, and several Gram-negative and Gram-positive bacteria. Complex 2c overcame metronidazole resistance in C. difficile at low micromolar dosages. In conclusion, the design of hybrid gold organometallic antibiotics might provide an useful strategy to obtain novel resistance-breaking metal-based antibacterial drug candidates with a favorable toxicity profile.
Experimental
General
The reagents were purchased from Sigma-Aldrich, Alfa Aesar or TCI and used without additional purification steps. All reactions were performed without precautions to exclude air or moisture. 1H and 13C NMR were recorded on a DRX-400 AS, AVIIIHD 500 or AVII 600. A Finnigan LCQDeca or a Finnigan MAT 95 was used to record the ESI mass spectra. The elemental analyses were performed using a Flash EA 1112 (Thermo Quest CE Instruments). Absorption measurements for TrxR inhibition and antiproliferative activity were performed on a Perkin-Elmer 2030 Multilabel Reader VICTORTM X4. Compound 1a [20] and 1-(ethoxycarbonylmethyl)-3-methylbenzimidazolium bromide [34] were prepared as described.
Chlorido-(1,3-diethyl-5-carboxybenzimidazol-2-ylidene)gold(I) (1b)
The compound was prepared according to a reported method with minor modifications [30]. Compound 1a (158.0 mg, 0.33 mmol) was suspended in 18 mL absolute ethanol and a volume of 18 mL NaOH (42.3 mg, 1.06 mmol) in purified water was added under constant stirring. The mixture was heated for 65 min under reflux conditions and directly afterwards the volume was reduced using a rotary evaporator. The pH of the mixture was set to 1–2 by using diluted hydroxylic acid, extracted with dichloromethane, and the combined organic phases were dried over sodium sulphate. The volume was reduced using a rotary evaporator, the remaining solution was overlayed with n-hexane, and the precipitate formed over time was isolated, washed with n-pentane, and dried at 40 °C under reduced pressure. yield: 71.2 mg (0.158 mmol, 48%), 1H-NMR (400.4 MHz, DMSO-d6): δ [ppm] = 13.29 (s, 1H, COOH), 8.38 (d, J = 1.4 Hz, 1H, C(Ar)H), 8.06 (dd, J = 8.6 Hz, 1.4 Hz, 1H, C(Ar)H), 7.95 (d, J = 8.6 Hz, 1H, C(Ar)H), 4.62–4.51 (m, 4H, NCH2CH3), 1.46 (t, J = 7.2 Hz, 6H, NCH2CH3); 13C-NMR (100.7 MHz, DMSO-d6): δ [ppm] = 178.5 (1C, NCAuN), 166.8 (1C, COOH), 135.0 (1C, C(Ar)), 132.1 (1C, C(Ar)), 127.0 (1C, C(Ar)), 125.5 (1C, C(Ar)H), 113.5 (1C, C(Ar)H), 112.1 (1C, C(Ar)H), 43.6 (1C, NCH2CH3), 43.5 (1C, NCH2CH3), 15.5 (1C, NCH2CH3), 15.3 (1C, NCH2CH3).; MS (ESI) m/z = 437.4 [M-Cl–H+Na]+, 456.2 [M-Cl + MeCN]+; CHN(calc./found): C 31.98/32.02, H 3.13/3.13, N 6.22/6.01.
Chlorido-(1,3-diethyl-5-((2-(2-methyl-5-nitroimidazol-1-yl)ethoxy)carbonyl)benzimidazol-2-ylidene)gold(I) 1c
1b (111.6 mg, 0.248 mmol), 4-dimethylaminopyridine (10.6 mg, 0.087 mmol) and metronidazole (40.3 mg, 0.235 mmol) were suspended in 0.5 mL dimethylformamide and stirred for 10 min at room temperature. A solution of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC, 45.6 mg, 0.238 mmol) in 0.5 mL DMF was added, the mixture was stirred for 78 h, poured in distilled water, and stirred for 15 min. The formed precipitate was collected by filtration, washed with distilled water, and dried at 40 °C under reduced pressure. Yield: 79.5 mg (0.132 mmol, 53%); 1H-NMR (500.3 MHz, DMSO-d6): δ [ppm] = 8.24 (d, J = 1.4 Hz, 1H, C(Ar)H), 8.06 (s, 1H, C(Im)H), 7.99 (d, J = 8.6 Hz, 1H, C(Ar)H), 7.94 (dd, J = 8.6 Hz, 1.4 Hz, 1H, C(Ar)H), 4.83–4.75 (m, 2H, NCH2CH2O), 4.75–4.65 (m, 2H, NCH2CH2O), 4.60–4.51 (m, 4H, NCH2CH3), 2.49 (s, 3H, NCCH3N), 1.49–1.43 (m, 6H, NCH2CH3); 13C-NMR (125.8 MHz, DMSO-d6): δ [ppm] = 179.0 (1 C, NCAuN), 164.8 (1 C, COO), 151.5 (1 C, NCCH3N), 138.6 (1 C, C(Im)NO2), 135.5 (1 C, C(Ar)), 133.2 (1 C, C(Ar)), 132.1 (1 C, C(Im)H), 125.2 (1 C, C(Ar)), 125.1 (1 C, C(Ar)H), 113.3 (1 C, C(Ar)H), 112.5 (1 C, C(Ar)H), 63.2 (1 C, NCH2CH2O), 44.6 (1 C, NCH2CH2O), 43.7 (1 C, NCH2CH3), 43.6 (1 C, NCH2CH3), 15.5 (1 C, NCH2CH3), 15.4 (1 C, NCH2CH3), 13.9 (1 C, NCCH3N); MS (ESI) m/z = 626.1 [M + Na]+, 939.3 [2 M-AuCl2]+, 1171.2 [2 M-Cl]+, 1229.2 [2 M + Na]+; CHN (calc./found) C 35.81/35.88, H 3.51/3.63, N 11.60/11.25.
Chlorido-(1-(2-ethoxy-2-oxoethyl)-3-methylbenzimidazol-2-ylidene)gold(I) 2a
1-(Ethoxycarbonylmethyl)-3-methylbenzimidazolium bromide (300.0 mg, 0.369 mmol) and silver oxide (51.2 mg, 0.221 mmol) in dichloromethane (20 mL) were stirred over night in the dark at room temperature. Chlorido(dimethylsulfide)gold(I) (119.6 mg, 0.406 mmol) was added, the mixture was stirred for another 40 h at room temperature, filtered using Celite®, the solvent was evaporated and the product was dried at 40 °C under reduced pressure. Yield: 413.4 mg (0.917 mmol, 91%); 1H-NMR (600.1 MHz, DMSO-d6): δ [ppm] = 7.84–7.76 (m, 2H, C(Ar)H), 7.55–7.46 (m, 2H, C(Ar)H), 5.43 (s, 2H, NCH2CO2), 4.21 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.05 (s, 3H, NCH3), 1.24 (t, J = 7.1 Hz, 3H, OCH2CH3); 13C-NMR (150.9 MHz, DMSO-d6): δ [ppm] = 178.4 (1C, NCAuN), 167.4 (1C, CO2), 133.0 (1C, C(Ar)), 132.9 (1C, C(Ar)), 124.6 (1C, C(Ar)H), 124.4 (1C, C(Ar)H), 112.1 (1C, C(Ar)H), 112.0 (1C, C(Ar)H), 61.5 (1C, OCH2CH3), 49.0 (1C, NCH2CO2), 35.0 (1C, NCH3), 13.9 (1C, OCH2CH3); MS (ESI +) m/z = 473.0 [M + Na]+, 633.2 [2 M-AuCl2]+, 923.1 [2 M + Na]+; elemental analysis (calc./found) C 31.98/31.81, H 3.13/3.09, N 6.22/6.10.
Chlorido-(1-carboxymethyl-3-methyl)benzimidazol-2-ylidene)gold(I) 2b
Compound 2a (242.6 mg, 0.538 mmol) was suspended in 20 mL absolute ethanol and a volume of 20 mL NaOH (72.3 mg, 1.808 mmol) in purified water was added under constant stirring. The mixture was heated for 65 min under reflux conditions and directly afterwards the volume was reduced using a rotary evaporator. The pH of the mixture was set to 2–3 by using diluted hydrochloric acid, extracted with dichloromethane, and the combined organic phases were dried over sodium sulphate. The volume was reduced to roughly 30–40% using a rotary evaporator, the remaining solution was overlayed with n-hexane, and the precipitate formed over time was isolated, washed with n-pentane, and dried at 40 °C under reduced pressure. Yield: 105.2 mg (0.249 mmol, 46%); 1H-NMR (500.3 MHz, DMSO-d6): δ [ppm] = 7.82–7.76 (m, 2H, C(Ar)H), 7.52–7.46 (m, 2H,C(Ar)H), 5.30 (s, 2H, NCH2), 4.04 (s, 3H, NCH3); 13C-NMR (125.8 MHz, DMSO-d6): δ [ppm] = 178.3 (1C, NCAuN), 168.6 (1C, COOH), 133.1 (1C,C(Ar)), 133.0 (1C, C(Ar)), 124.5 (1C, C(Ar)H), 124.3 (1C, C(Ar)H), 112.0 (2C, C(Ar)H), 49.2 (1C, NCH2), 35.0 (1C, NCH3); MS (ESI) m/z = 444.8 [M + Na]+, 808.7 [2 M-Cl]+, 830.7 [2 M + Na–H-Cl]+, 852.6 [2 M + 2Na-2H-Cl]+; CHN (calc./found) C 28.42/28.62, H 2.39/2.33, N 6.63/6.32.
Chlorido-(1-methyl-3-(2-(2-(2-methyl-5-nitroimidazol-1-yl)ethoxy)-2-oxoethyl)-benzimidazol-2-yliden)gold(I) 2c
2b (147.1 mg, 0.348 mmol), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC, 64.1 mg, 0.334 mmol), 4-dimethylaminopyridine (14.9 mg, 0.122 mmol) and metronidazole (56.6 mg, 0.331 mmol) were dissolved in 1.0 mL dimethlyformamide and stirred for 48 h at room temperature. The mixture was poured into 30 mL dichloromethane and washed with 25 mL of distilled water. The aqueous phase was extracted twice with 30 mL dichloromethane, the combined organic phases were dried over sodium sulphate, and the volume was reduced in a rotary evaporator to approximately 1 mL of a yellow solution. A volume of 3.0 mL dichloromethane was added, the solution was overlayed with n-hexane and left at − 20 °C. The precipitate was collected, washed with n-hexane, and purified by column chromatography over silica with dichloromethane/methanol (96/4) as eluent. Yield: 27.0 mg (0.047 mmol, 14%); 1H-NMR (500.3 MHz, DMSO-d6): δ [ppm] = 8.00 (s, 1H, NCHCNO2), 7.83 (dt, J = 8.1 Hz, 0.9 Hz, 1H, C(Ar)H), 7.71–7.65 (m, 1H, C(Ar)H), 7.57–7.45 (m, 2H, C(Ar)H), 5.42 (s, 2H, NCH2CO2), 4.58 (dd, J = 5.4 Hz, 4.2 Hz, 2H, OCH2CH2N), 4.52 (dd, J = 5.4 Hz, 4.1 Hz, 2H, OCH2CH2N), 4.05 (s, 3H, NCH3), 2.29 (s, 3H, NCCH3N); 13C-NMR (125.8 MHz, DMSO-d6): δ [ppm] = 178.4 (1C, NCAuN), 167.0 (1C, CH2CO2), 151.4 (1C, NCCH3N), 138.3 (1C, CNO2), 133.0 (2C, C(Met)H + C(Ar)), 132.7 (1C, C(Ar)), 124.6 (1C, C(Ar)H), 124.5 (1C, C(Ar)H), 112.2 (1C, C(Ar)H), 111.9 (1C, C(Ar)H), 63.9 (1C, NCH2CH2O), 48.9 (1C, NCH2CO2), 44.4 (1C, NCH2CH2O), 35.1 (1C, NCH3), 13.8 (1C, NCCH3N); MS (ESI +) m/z = 539.8 [M-Cl]+, 575.8 [M + H]+, 597.7 [M + Na]+; elemental analysis (calc./found) C 33.38/33.58, H 2.98/2.98, N 12.16/11.68.
Inhibition of bacterial TrxR (E. coli)
The TrxR (E.coli) inhibition assay was performed according to a previously published procedure [8]. It is partly based on the procedure developed by Lu et al.[35] and detects the formation of 5-TNB (5-thionitrobenzoic acid). Solutions of E. coli TrxR (35.4 U/mL) and of its substrate thioredoxin (Trx) E. coli (156 µg/mL) (both purchased from Sigma-Aldrich and diluted with distilled water) and fresh stock solutions of the test compounds (in DMF) were prepared (1c was administered as suspension). TE buffer (Tris–HCl 50 mM, EDTA 1 mM, pH 7.5) containing graded concentrations of the respective compounds (20 µL) or buffer without compounds (20 µL, as control) were mixed with TrxR solution (10 µL), Trx solution (10 µL), and a solution of NADPH (200 µM) in TE buffer (100 µL) in a well on a 96-well plate. As blank solution, 200 µM NADPH in TE buffer (100 µL) mixed with a DMF/buffer mixture (40 µL) was used (final concentrations of DMF: 0.5% v/v). The solutions on the 96-well plate were incubated for 75 min at 25 °C with moderate shaking. A volume of 100 µL of a reaction mixture (TE buffer containing 200 µM NADPH and 5 mM DTNB) was added to each well to initiate the reaction. After thorough mixing, the formation of 5-TNB was monitored by a microplate reader at 405 nm in 35 s intervals (10 measurements). The values were corrected by subtraction of the values for the blank solution. The increase in concentration of 5-TNB followed a linear trend (r2 ≥ 0.990) and the enzymatic activities were calculated as the gradients (increase in absorbance per second) thereof. Absence of interference with the assay components was confirmed by a negative control experiment for each test compound. The highest test compound concentration was used and the enzyme solution was replaced by TE buffer for this purpose. The IC50 values were calculated as the concentration of the compound decreasing the enzymatic activity of the positive control by 50% and are presented as the means and standard deviations of independent repeated experiments.
Cell culture
A549 lung carcinoma, HT-29 colon carcinoma, MDA-MB-231 breast cancer, and Vero E6 monkey kidney cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; 4.5 g/L D-glucose, l-glutamine, pyruvate), supplemented with fetal bovine serum superior, standardized (Biochrom GmbH, Berlin, 10% v/v) and gentamycin (50 mg/L) with a weekly passage.
Antiproliferative assay in tumorigenic and non-tumorgenic cells
The antiproliferative effects were determined according to a previously published procedure [20]. A volume of 100 µL of cell suspension of A549 cells, MDA-MB-231, HT-29 or Vero E6 cells was transferred into the wells of a 96-well plates and incubated at 37 °C under 5% CO2 for 72 h. Stock solutions of the compounds (1b, 1c, and 2c were suspended due to limited solubility) were freshly prepared in dimethylformamide (DMF) and diluted with the respective cell medium to obtain various concentrations (final concentration of DMF: 0.1% v/v). After 72 h (A549, HT-29, Vero E6) or 96 h (MDA-MB-231) of exposure, the biomass of the cells was determined via crystal violet staining and the IC50 value was determined as the concentration that caused 50% inhibition of cell proliferation relative to an untreated control. The results were calculated as the mean values of three independent experiments, unless stated otherwise.
Antibacterial effects against aerobic bacteria
The following strains were used and maintained at 37 °C in MHB (21 g/L Müller Hinton, pH 7.4) or TSY (30 g/L trypticase soy broth, 3 g/L yeast extract, pH 7.0–7.2) medium. Acinetobacter baumannii (DSM 30007, ATCC 19606) in MHB, Escherichia coli (DSM1116, ATCC 9637) in TSY, Klebsiella pneumoniae (DSM 11678, ATCC33495) in MHB, Pseudomonas aeruginosa PA7 (DSM 24068) in MHB, Enterococcus faecium (DSM 20477, ATCC 19434) in TSY, Staphylococcus aureus MRSA (DSM 11822, ICB 25701) in TSY). Minimum inhibitory concentration (MIC) values were determined following a standardized protocol in broth dilution assays. The compounds and auranofin were serially diluted (1b and 1c showed limited solubility) starting from 64 µg/ml using a pipetting robot (epMotion, Eppendorf, Germany). Starting inocula of 2–8 × 105 colony-forming units per ml in MHB or TSY media at 37 °C were used, and serial dilutions were carried out in 384-well microtiter plates in duplicate. After incubation of the plates for 20 h at 37 °C, the absorbance at 600 nm was measured to determine the MIC value (Enspire Multimode Microplate Reader, Perkin Elmer Inc.). The MIC values for the tested compounds were determined in three independent experiments by a curve-fitting procedure using the GraphPad Prism software (Graphpad Software, Inc.).
Antibacterial effects against C. difficile
The Clostridioides difficile strains 1296 T (DSM 1296), VPI10463 (ATCC 43255) and the metronidazole resistant strain IB136 (NCTC 14385)[33] (provided by Ulrich Nübel, DSMZ Braunschweig) were maintained in prereduced BHIS medium (37 g/L BHI, oxoid, 5% yeast extract, 1% L-cysteine) in a Coy-Laboratories anaerobic chamber (85% N2, 10% CO2, 5% H2) at 37 °C. Freshly prepared cultures were incubated until the exponential growth phase (OD600 = 0.2–0.4) and were adjusted to 1 × 106 cfu/mL before inoculation. The test compounds were dissolved in DMSO and by two-fold dilution steps concentrations in the range from 200 µM to 0.39 µM in BIHS medium were obtained. The solutions (100µL) were added to 100 µL of a diluted C. difficile culture (total volume: 200 µL in BIHS, 5 × 105 C. difficile, 100–0.195 µM test compound per well). The OD600-values over a period of 48 h were measured with a LogPhase 600 4-plate reader (BioTek) and compared with blank BHIS.
References
Antimicrobial Resistance Collaborators (2022) Lancet 399:629–655. DOI. https://doi.org/10.1016/S0140-6736(21)02724-0
Biegański P, Szczupak Ł, Arruebo M, Kowalski K (2021) RSC Chem Biol 2:368–386. https://doi.org/10.1039/d0cb00218f
Frei A, Verderosa AD, Elliott AG, Zuegg J, Blaskovich MAT (2023) Nat Rev Chem 7:202–224. https://doi.org/10.1038/s41570-023-00463-4
Waters JE, Stevens-Cullinane L, Siebenmann L, Hess J (2023) Curr Opin Microbiol 75:102347. https://doi.org/10.1016/j.mib.2023.102347
Yeo CI, Goh CHP, Tiekink ER, Chew J (2024). Coord Chem Rev. https://doi.org/10.1016/j.ccr.2023.215429
Liu Y, Lu Y, Xu Z, Ma X, Chen X, Liu W (2022) Drug Disc Today 27:1961–1973. https://doi.org/10.1016/j.drudis.2022.02.010
Sun H, Zhang Q, Wang R, Wang H, Wong Y-T, Wang M, Hao Q, Yan A, Kao RY-T, Ho P-L, Li H (2020) Nat Commun 11:5263. https://doi.org/10.1038/s41467-020-18939-y
Schmidt C, Karge B, Misgeld R, Prokop A, Franke R, Brönstrup M, Ott I (2017) Chem Eur J 23:1869–1880. https://doi.org/10.1002/chem.201604512
Marques A, Carabineiro SAC, Aureliano M, Faleiro L (2023). Toxics. https://doi.org/10.3390/toxics11110879
Zhang Q, Wang M, Hu X, Yan A, Ho P-L, Li H, Sun H (2023) J Biol Inorg Chem 28:225–234. https://doi.org/10.1007/s00775-022-01983-y
Ratia C, Ballén V, Gabasa Y, Soengas RG, Velasco-de Andrés M, Iglesias MJ, Cheng Q, Lozano F, Arnér ESJ, López-Ortiz F, Soto SM (2023) Front Microbiol 14:1198473. https://doi.org/10.3389/fmicb.2023.1198473
Mármol I, Quero J, Azcárate P, Atrián-Blasco E, Ramos C, Santos J, Gimeno MC, Rodríguez-Yoldi MJ, Cerrada E (2022). Pharmaceutics. https://doi.org/10.3390/pharmaceutics14102064
Gascón E, Otal I, Maisanaba S, Llana-Ruiz-Cabello M, Valero E, Repetto G, Jones PG, Oriol L, Jiménez J (2022) Dalton Trans 51:13657–13674. https://doi.org/10.1039/d2dt01963a
Carpio-Granillo M, Zuno-Cruz FJ, Sánchez-Cabrera G, Rojo-Gómez EG, González-Ábrego DO, Coronel-Olivares C, Caviedes MF, Andrade-López N, Rosales-Hoz MJ, Leyva MA (2022). Polyhedron. https://doi.org/10.1016/j.poly.2022.115726
Ratia C, Cepas V, Soengas R, Navarro Y, Velasco-de Andrés M, Iglesias MJ, Lozano F, López-Ortiz F, Soto SM (2022). Front Microbiol. https://doi.org/10.3389/fmicb.2022.815622
Mather JC, Wyllie JA, Hamilton A, Da Soares Costa TP, Barnard PJ (2022) Dalton Trans 51:12056–12070. https://doi.org/10.1039/d2dt01657e
Chakraborty P, Oosterhuis D, Bonsignore R, Casini A, Olinga P, Scheffers D-J (2021) ChemMedChem 16:3060–3070. https://doi.org/10.1002/cmdc.202100342
Marzo T, Cirri D, Pollini S, Prato M, Fallani S, Cassetta MI, Novelli A, Rossolini GM, Messori L (2018) ChemMedChem 13:2448–2454. https://doi.org/10.1002/cmdc.201800498
Cassetta MI, Marzo T, Fallani S, Novelli A, Messori L (2014) Biometals 27:787–791. https://doi.org/10.1007/s10534-014-9743-6
Büssing R, Karge B, Lippmann P, Jones PG, Brönstrup M, Ott I (2021) ChemMedChem 16:3402–3409. https://doi.org/10.1002/cmdc.202100381
Mahdavi SM, Bockfeld D, Büssing R, Karge B, Bannenberg T, Frank R, Brönstrup M, Ott I, Tamm M (2024) Dalton Trans 53:1942–1946. https://doi.org/10.1039/d3dt04135b
Harbut MB, Vilchèze C, Luo X, Hensler ME, Guo H, Yang B, Chatterjee AK, Nizet V, Jacobs WR, Schultz PG, Wang F (2015) Proc Nat Acad Sci USA 112:4453–4458. https://doi.org/10.1073/pnas.1504022112
Schmidt C, Zollo M, Bonsignore R, Casini A, Hacker SM (2022) Chem Commun 58:5526–5529. https://doi.org/10.1039/d2cc01259f
Schmidt C, Albrecht L, Balasupramaniam S, Misgeld R, Karge B, Brönstrup M, Prokop A, Baumann K, Reichl S, Ott I (2019) Metallomics 11:533–545. https://doi.org/10.1039/c8mt00306h
Schmidt C, Karge B, Misgeld R, Prokop A, Brönstrup M, Ott I (2017) Med Chem Commun 8:1681–1689. https://doi.org/10.1039/C7MD00269F
Giacomazzo GE, Conti L, Fagorzi C, Pagliai M, Andreini C, Guerri A, Perito B, Mengoni A, Valtancoli B, Giorgi C (2023) Inorg Chem 62:7716–7727. https://doi.org/10.1021/acs.inorgchem.3c00214
Eugene-Osoikhia TT, Badmus TO, Ayeni F (2020) Chem Search J 11:74–82
Szczupak Ł, Kowalczyk A, Trzybiński D, Woźniak K, Mendoza G, Arruebo M, Steverding D, Stączek P, Kowalski K (2020) Dalton Trans 49:1403–1415. https://doi.org/10.1039/c9dt03948a
Lewandowski EM, Szczupak Ł, Kowalczyk A, Mendoza G, Arruebo M, Jacobs LMC, Stączek P, Chen Y, Kowalski K (2020) ChemBioChem 21:2187–2195. https://doi.org/10.1002/cbic.202000054
Salorinne K, Man RWY, Li C-H, Taki M, Nambo M, Crudden CM (2017) Angew Chem Int Ed 56:6198–6202. https://doi.org/10.1002/anie.201701605
Babu T, Sarkar A, Karmakar S, Schmidt C, Gibson D (2020) Inorg Chem 59:5182–5193. https://doi.org/10.1021/acs.inorgchem.0c00445
Schmidt C, Babu T, Kostrhunova H, Timm A, Basu U, Ott I, Gandin V, Brabec V, Gibson D (2021) J Med Chem 64:11364–11378. https://doi.org/10.1021/acs.jmedchem.1c00706
Boekhoud IM, Hornung BVH, Sevilla E, Harmanus C, Bos-Sanders IMJG, Terveer EM, Bolea R, Corver J, Kuijper EJ, Smits WK (2020) Nat Commun 11:598. https://doi.org/10.1038/s41467-020-14382-1
Wang B, Hu J, Zhang X, Hu Y, Hu H (2000) J Heterocycl Chem 37:1533–1537. https://doi.org/10.1002/jhet.5570370620
Lu J, Vlamis-Gardikas A, Kandasamy K, Zhao R, Gustafsson TN, Engstrand L, Hoffner S, Engman L, Holmgren A (2013) FASEB J 27:1394–1403. https://doi.org/10.1096/fj.12-223305
Acknowledgements
Financial support by the DFG (Deutsche Forschungsgemeinschaft, bilateral grant BR 3572/4-1, for TU-BS and HZI) and the Lower Saxony Ministry for Science and Culture for the doctoral program "Drug Discovery and Cheminformatics for New Anti-Infectives (iCA)" are gratefully acknowledged.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Büssing, R., Bublitz, A., Karge, B. et al. An organometallic hybrid antibiotic of metronidazole with a Gold(I) N-Heterocyclic Carbene overcomes metronidazole resistance in Clostridioides difficile. J Biol Inorg Chem (2024). https://doi.org/10.1007/s00775-024-02064-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00775-024-02064-y