Abstract
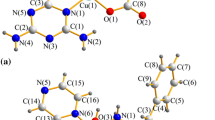
Four copper(II) coordination compounds from 2-benzimidazole propionic acid (Hbzpr) and 4-(benzimidazol-2-yl)-3-thiobutanoic acid (Hbztb) were synthesized and fully characterized by elemental analyses, electronic spectroscopy, FT-IR and mass spectrometry. The molecular structure for the four complexes was confirmed by single-crystal X-ray crystallography. The DNA-interacting properties of the two trinuclear and two mononuclear compounds were investigated using different spectroscopic techniques including absorption titration experiments, fluorescence spectroscopy and circular dichroism spectroscopy. Trinuclear [Cu3(bzpr)4(H2O)2](NO3)2·3H2O·CH3OH (2) and [Cu3(bzpr)4Cl2]·3H2O (3) bind to DNA through non-intercalative interactions, while for mononuclear [Cu(bzpr)2(H2O)]·2H2O (1) and [Cu(bztb)2]·2H2O (4), at minor concentrations in relation to the DNA, a groove binding interaction is favored, while at higher concentrations an intercalative mode is preferred. The nuclease properties of all complexes were studied by gel electrophoresis, which showed that they were able to cleave supercoiled plasmid DNA (form I) to the nicked form (form II). Compound 4 is even capable of generating linear form III (resulting from double-strand cleavage). The proposed mechanism of action involves an oxidative pathway (Fenton-type reaction), which produces harmful reactive species, like hydroxyl radicals.
Graphical abstract





















Similar content being viewed by others
References
Tullius TD (1989) Metal-DNA chemistry. ACS Symposium Series, vol 402. American Chemical Society, Washington DC
Loehrer PJ, Einhorn LH (1984) Ann Intern Med 100:704–713
Lippert B (1999) Cisplatin: chemistry and biochemistry of a leading anticancer drug. VHCA & Wiley-VCH, Zurich
Cuello-Garibo JA, James CC, Siegler MA, Bonnet S (2017) Chem Sq 1:2
Stern BR (2010) J Toxicol Environ Health Part A 73:114–127
Lippard SJ, Berg JM (1994) Principles of bioinorganic chemistry. Mill Valley, California
Jagadeesh M, Kalangi SK, Krishna LS, Reddy AV (2014) Spectrochim Acta, Part A 118:552–556
Sayen S, Carlier A, Tarpin M, Guillon E (2013) J Inorg Biochem 120:39–43
Duff B, Thangella VR, Creaven BS, Walsh M, Egan DA (2012) Eur J Pharmacol 689:45–55
Ali I, Wani WA, Saleem K, Hseih M-F (2013) Polyhedron 56:134–143
Li G-Y, Du K-J, Wang J-Q, Liang J-W, Kou J-F, Hou X-J, Ji L-N, Chao H (2013) J Inorg Biochem 119:43–53
Silveira VC, Benezra H, Luz JS, Georg RC, Oliveira CC, Ferreira AMC (2011) J Inorg Biochem 105:1692–1703
Patel MN, Dosi PA, Bhatt BS, Thakkar VR (2011) Spectrochim Acta Part A 78:763–770
Kellett A, Howe O, Connor MO, McCann M, Creaven BS, McClean S, Kia AF-A, Casey A, Devereux M (2012) Free Radical Biol Med 53:564–576
Kashanian S, Khodaei MM, Roshanfekr H, Shahabadi N, Mansouri G (2012) Spectrochim Acta Part A 86:351–359
Grau J, Renau C, Caballero AB, Caubet A, Pockaj M, Lorenzo J, Gamez P (2018) Dalton Trans 47:4902–4908
Schreiber JP, Deune M (1969) Biopolymers 8:139–152
Chikira M, Inue M, Negane R, Harada W, Shindo H, Antholine WE (2000) J Inorg Biochem 78:243–249
Morrow JR, Iranzo O (2004) Curr Opin Chem Biol 8:192–200
Erxleben A Interactions of copper complexes with nucleic acids
Spingler B, Da Pieve C (2005) Dalton Trans 1637–1643
Medina-Molner A, Rohner M, Pandiarajan D, Spingler B (2015) Dalton Trans 44:3664–3672
Harada W, Nojima T, Shibayama A, Ueda H, Sindo H, Chikira M (1996) J Inorg Biochem 64:273–285
Negane R, Chikira M, Oumi M, Shindo H, Antholine WE (2000) J Inorg Biochem 78:243–249
Li D-D, Tian J-L, Gu W, Liu X, Zeng H-H, Yan S-P (2011) J Inorg Biochem 105:894–901
Suntharalingam K, White AJP, Vilar R (2010) Inorg Chem 49:8371–8380
Suntharalingam K, Hunt DJ, Duarte AA, White AJP, Mann DJ, Vilar R (2012) Chem Eur J 41:4955–4965
Skinnerm WA, Schelstraete MGM, Baker BR (1959) J Org Chem 24:1827
Biron KK (2006) Antivir Res 71:154–163
Middleton T, Lim HB, Montgomery D, Rockway T, Tang H, Cheng X, Lu L, Mo H, Kohlbrenner WE, Molla A, Kati WM (2004) Antivir Res 64:35–45
Labanauskas L, Brukštus A, Udre˙ naite˙ E, Gaidelis P, Bucˇinskaite˙ V (2003) Chemija (Vilnius) 14:49
Sari H, Covington AK (2005) J Chem Eng Data 50:1425–1429
Meaney M, Allister J, McKinstry B, McLaughlin K, Brennan GP, Forbes AB, Fairweather I (2007) Parasitol Res 100:1091–1104
Mirskova AN, Levkovskaya GG, Mirskov RG, Voronkov MG (2008) Russ J Org Chem 44:1478–1485
Luneau D, Rey P (2005) Coord Chem Rev 249:2591–2611
Kabatc J, Jurek K (2012) Polymer 53:1973–1980
Yoe F, Flores-Álamo M, Morales F, Escudero R, Cortés-Hernández H, Castro M, Barba-Behrens N (2014) Inorg Chim Acta 423:36–45
Sheldrick GM (2015) Acta Cryst. A71:3–8
Hübschle CB, Sheldrick GM, Dittrich B (2011) J Appl Crystallogr 44:1281–1284
Spek AL (2015) Acta Cryst. C71:9–18
Reichmann MF, Rice SA, Thomas CA, Doty P (1954) J Am Chem Soc 76:3047–3053
Wolfe A, Shimer GHJ, Meehan T (1987) Biochemistry 26:6392–6396
Lakowicz JR, Weber G (1973) Biochemistry 12(21):4161–4170
Shubsda MF, Goodisman J, Dabrowiak JC (1997) J Biochem Biophys Methods 34:73–79
Patel MN, Dosi PA, Bhatt BS (2010) Polyhedron 29:3238–3245
Lever ABP (1968) J Chem Ed 45:711–712
Valderrama-Negrón AC, Alves WA, Cruz ÁS, Rogero SO, de Oliveira Silva D (2011) Inorg Chim Acta 367:58–92
Nakamoto K (1986) Infrared and Raman Spectra of Inorganic and Coordination Compounds. Wiley-Interscience
Addison AW, Rao TN, Reedijk J, van Rijn J, Verschoor GCJ (1984) Chem Soc 7:1349–1356
Klein A, Neugebauer E, Krest A, Lüning A, Garbe S, Arefyeva N, Schlörer N (2015) Inorganics 3:118–138
Jahn HA, Teller E (1937) Proc R Soc Lond Ser A161:220–235
Matthews CJ, Heath SL, Elsegood MRJ, Clegg W, Leese TA, Lockhart JC (1998) J Chem Soc Dalton Trans 12:1973–1977
Kelly JM, Tossi AB, McConnell DJ, OhUigin C (1985) Nucleic Acids Res 13:6017–6034
Meenongwa A, Chaveerach U, Siriwong K (2011) Inorg Chim Acta 366:357–365
Chaveerach U, Meenongwa A, Trongpanich Y, Soikum C, Chaveerach P (2010) Polyhedron 29:731–738
Marmur J (1961) J Mol Biol 3:208–218
Meenongwa A, Brissos RF, Soikum C, Chaveerach P, Gamez P, Trongpanich Y, Chaveerach U (2015) N J Chem 39:664–675
Nyarko E, Hanada N, Habib A, Tabata M (2004) Inorg Chim Acta 357:739–745
Silveira VC, Benezra H, Luz JS, Georg RC, Oliveira CC, Ferreira AMC (2011) J Inorg Biochem 105:1692–1703
Ling X, Zhong W, Huang Q, Ni K (2008) J Photochem Photobiol B 93:172–176
Ivanov VI, Minchenkova LE, Schyolkina AK, Poletayev AI (1973) Biopolymers 12:89–110
Collins CH, Lyne PM (1970) Microbiological methods. University Park Press, Baltimore
Sigman DS, Chen C-HB (1986) Acc Chem Res 19:180–186
Acknowledgements
The financial support from CONACYT, grant CB2012-178851 and DGAPA-UNAM for grant IN224516 is acknowledged. V.A.B.-G. thanks a CONACYT scholarship. P.G. acknowledges the financial support from the Ministerio de Ciencia, Innovación y Universidades (projects CTQ2015-70371-REDT and CTQ2017-88446-R AEI/FEDER, UE). We thank P. Fierro for technical support.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Barrera-Guzmán, V.A., Rodríguez-Hernández, E.O., Ortíz-Pastrana, N. et al. Efficient copper-based DNA cleavers from carboxylate benzimidazole ligands. J Biol Inorg Chem 23, 1165–1183 (2018). https://doi.org/10.1007/s00775-018-1598-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-018-1598-9