Abstract
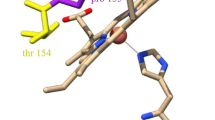
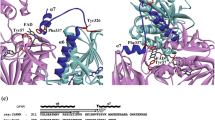
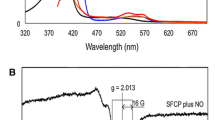
Cytochromes c’, that occur in methanotrophic, denitrifying and photosynthetic bacteria, form unusual proximal penta-coordinate NO complexes via a hexa-coordinate distal NO intermediate. Their NO binding properties are similar to those of the eukaryotic NO sensor, soluble guanylate cyclase, for which they provide a valuable structural model. Previous studies suggested that hydrogen bonding between the displaced proximal histidine (His120) ligand (following its dissociation from heme due to trans effects from the distally bound NO) and a conserved aspartate residue (Asp121) could play a key role in allowing proximal NO binding to occur. We have characterized three variants of Alcaligenes xylosoxidans cytochrome c’ (AXCP) where Asp121 has been replaced by Ala, Ile and Gln, respectively. In all variants, hydrogen bonding between residue 121 and His120 is abolished yet 5-coordinate proximal NO species are still formed. Our data therefore demonstrate that the His120–Asp121 bond is not essential for proximal NO binding although it likely provides an energy minimum for the displaced His ligand. All variants have altered proximal pocket structure relative to native AXCP.






Similar content being viewed by others
Abbreviations
- CytCp:
-
Cytochrome c’
- AXCP:
-
Alcaligenes xylosoxidans cytochrome c’
- SFCP:
-
Shewanella frigidimarina cytochrome c’
- SLS:
-
Swiss Light Source
- 5c:
-
5-Coordinate
- 6c:
-
6-Coordinate
References
Romao MJ, Archer AM (2006) Handbook of Metalloproteins 1. Wiley, Hoboken
Lawson DM, Stevenson CE, Andrew CR, Eady RR (2000) EMBO J 19:5661–5671
Andrew CR, Green EL, Lawson DM, Eady RR (2001) Biochemistry 40:4115–4122
Moir JW (1999) Biochim Biophys Acta 1430:65–72
Cross R, Aish J, Paston SJ, Poole RK, Moir JW (2000) J Bacteriol 182:1422–1447
Martin E, Berka V, Sharina I, Tsai AL (2012) Biochemistry 51:2737–2746
Silkstone G, Kapetanaki SM, Husu I, Vos MH, Wilson MT (2010) J Biol Chem 285:19785–19792
Vicente JB, Colaço HG, Mendes MIS, Sarti P, Leandro P, Giuffrè A (2014) J Biol Chem 289:8579–8587
Cutruzzolà F, Arcovito A, Giardina G, della Longa S, D’Angelo P, Rinaldo S (2014) Biometals 27:763–773
Andrew CR, George SJ, Lawson DM, Eady RR (2002) Biochemistry 41:2353–2360
Kruglik SG, Lambry JC, Cianetti S, Martin JL, Eady RR, Andrew CR, Negrerie M (2007) J Biol Chem 282:5053–5062
Marti MA, Capece L, Crespo A, Doctorovich F, Estrin DA (2005) J Am Chem Soc 127:7721–7728
Barbieri S, Murphy LM, Sawers RG, Eady RR, Hasnain SS (2008) J Biol Inorg Chem 13:531–540
Hough MA, Antonyuk SV, Barbieri S, Rustage N, McKay AL, Servid AE, Eady RR, Andrew CR, Hasnain SS (2011) J Mol Biol 405:395–409
Manole A, Kekilli D, Svistunenko DA, Wilson MT, Dobbin PS, Hough MA (2015) J Biol Inorg Chem 20:675–686
Antonyuk SV, Rustage N, Petersen CA, Arnst JL, Heyes DJ, Sharma R, Berry NG, Scrutton NS, Eady RR, Andrew CR, Hasnain SS (2011) Proc Natl Acad Sci USA 108:15780–15785
Kekilli D, Dworkowski FS, Pompidor G, Fuchs MR, Andrew CR, Antonyuk S, Strange RW, Eady RR, Hasnain SS, Hough MA (2014) Acta Crystallogr D Biol Crystallogr 70:1289–1296
Arslan E, Schulz H, Zufferey R, Kunzler P, Thony-Meyer L (1998) Biochem Biophys Res Commun 251:744–747
Yoshimura T, Suzuki S, Nakahara A, Iwasaki H, Masuko M, Matsubara T (1986) Biochemistry 25:2436–2442
Otwinowski Z, Minor W (1997) Method Enzymol 276:307–326
Vagin A, Teplyakov A (2010) Acta Crystallogr D Biol Crystallogr 66:22–25
Murshudov GN, Vagin AA, Dodson EJ (1997) Acta Crystallogr D Biol Crystallogr 53:240–255
Potterton E, Briggs P, Turkenburg M, Dodson E (2003) Acta Crystallogr D Biol Crystallogr 59:1131–1137
Emsley P, Lohkamp B, Scott WG, Cowtan K (2010) Acta Crystallogr D Biol Crystallogr 66:486–501
Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) J Appl Crystallogr 26:283–291
Davis IW, Leaver-Fay A, Chen VB, Block JN, Kapral GJ, Wang X, Murray LW, Arendall WB 3rd, Snoeyink J, Richardson JS, Richardson DC (2007) Nucleic Acids Res 35:W375–W383
Sheldrick G (2008) Acta Crystallogr A 64:112–122
Pompidor G, Dworkowski FSN, Thominet V, Schulze-Briese C, Fuchs MR (2013) J Synchrotron Radiat 20:765–776
Dworkowski FS, Hough MA, Pompidor G, Fuchs MR (2015) Acta Crystallogr D Biol Crystallogr 71:27–35
Yoshimura T, Fujii S, Kamada H, Yamaguchi K, Suzuki S, Shidara S, Takakuwa S (1996) Biochim Biophys Acta 1292:39–46
Acknowledgments
D.K. was supported by a School Studentship at the University of Essex. X-ray diffraction data were measured at the Swiss Light Source under long-term award 20111166 to M.H. and funded in part via BioStructX award 2370 to M.H & R.W.S. R.W.S acknowledges financial support from a Wellcome Trust Fellowship Award. We acknowledge the assistance of Dr Svetlana Antonyuk for assistance in the use of Shelx for esd determination, Dr Andrey Lebedev with restraint libraries in refinement and Alex Chivu for assistance with protein preparation.
Author information
Authors and Affiliations
Corresponding author
Additional information
D. D. Ghafoor and D. Kekilli contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghafoor, D.D., Kekilli, D., Abdullah, G.H. et al. Hydrogen bonding of the dissociated histidine ligand is not required for formation of a proximal NO adduct in cytochrome c’. J Biol Inorg Chem 20, 949–956 (2015). https://doi.org/10.1007/s00775-015-1278-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-015-1278-y